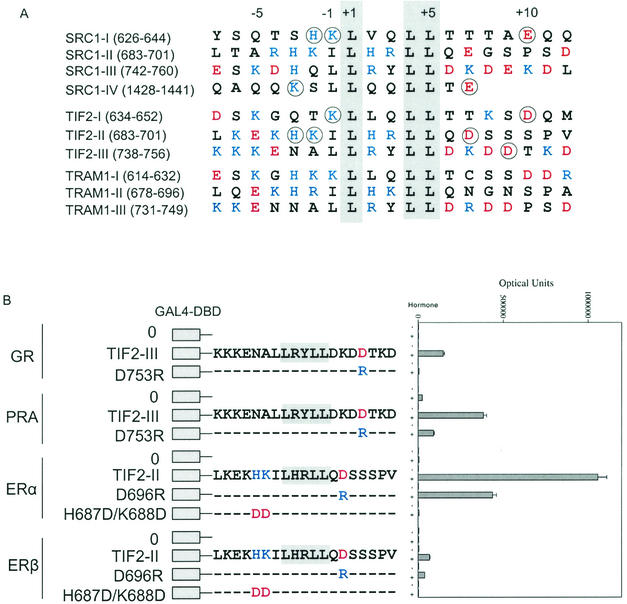
FIG. 4.
Flanking charged residue requirements of p160 coactivator LXXLL motif binding to steroid receptors. (A) Amino acid sequence of the LXXLL motif regions in the human p160 coactivators SRC1 (47), TIF2 (29, 56), and TRAM1 (53). Basic residues K, R, and H are shown in blue, and acidic residues D and E are shown in red. The conserved LXXLL motif is shaded, and the residues that were mutated are circled. The LXXLL motifs are numbered relative to the start of the core motif. (B) Two-hybrid interaction assay of TIF2-LXXLL peptides II and III with full-length steroid receptors. GAL fusion peptide vectors coding for TIF2-LXXLL-II and TIF2-LXXLL-III were cotransfected with the 5XGAL4Luc3 reporter vector and expression vectors for GR (pCMVhGR), PR-A (VP-PR-A), ERα (VP-ERα-LBD coding for residues 312 to 595), and ERβ (VP-ERβ full-length residues 1 to 530). Transfected HepG2 cells were incubated in the absence or presence of 10 nM dexamethasone for GR, 10 nM R5020 for PR-A, and 1 μM 17β-estradiol for ERα and ERβ.