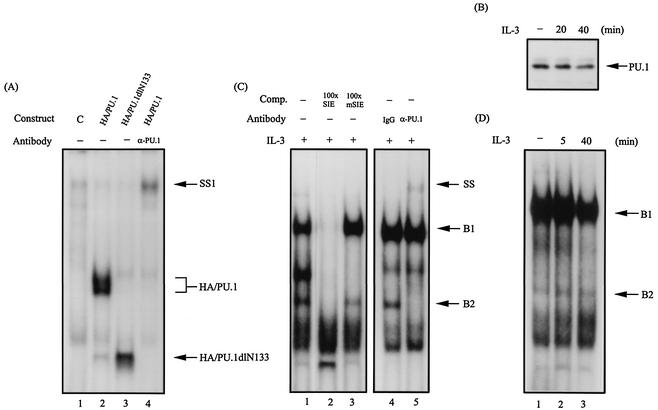
FIG. 1.
PU.1 is one component of the SIE-binding complex. (A) Gel shift assay with the 32P-labeled SIE probe using in vitro-translated HA/PU.1 (lane 2) or HA/PU.1dlN133 (lane 3). Lane 4, same as lane 2 except that the anti-PU.1 antibody was included in the assay. Lane 1 is the result of a control experiment using the reticulocyte lysates programmed with an empty expression vector. SS1, the HA/PU.1-DNA complex supershifted by the PU.1 antibody. (B) PU.1 is present in the complex pulled down by the SIE-containing oligonucleotide. The oligonucleotide pulldown assay using lysates of Ba/F3 cells with or without prior stimulation with IL-3 was carried out as described in Materials and Methods. The protein complex pulled down by this assay was analyzed by Western blotting with an antibody specific for PU.1. (C) Lane 1, same as lane 2 of panel A, except that nuclear extracts from IL-3-stimulated Ba/F3 cells were used in the gel shift assay. Lanes 2 and 3 are results of the same experiment with the inclusion of a 100-fold molar excess of unlabeled wild-type SIE- and mSIE (see Materials and Methods for the bases changed in the nucleotide sequence)-containing oligonucleotides, respectively, as a cold competitor (Comp.). SS denotes the B2 complex supershifted by the PU.1 antibody. (D) Same as lane 1 of panel C, except that the assay was carried out with nuclear extracts from Ba/F3 cells with or without IL-3 treatments.