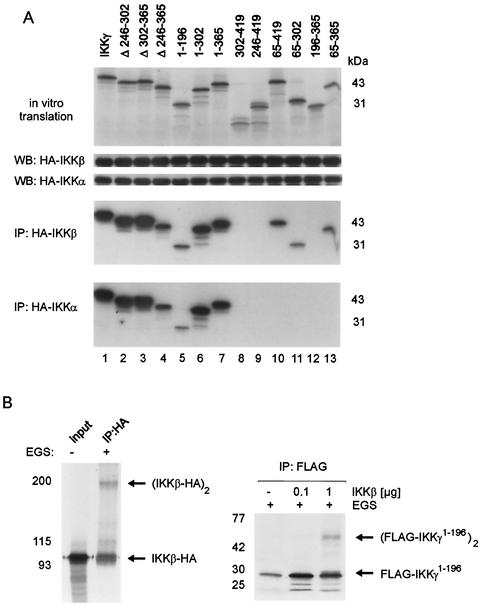
FIG. 5.
Physical interaction of IKKα and IKKβ with IKKγ involves overlapping but distinct N-terminal domains of IKKγ and does not require IKKγ tetramerization. (A) [35S]methionine-labeled IKKγ mutants were prepared by coupled in vitro transcription and translation. Labeling efficiency was controlled by SDS-PAGE or autoradiography (top gel). 293 cells were transiently transfected with HA-tagged IKKα (HA-IKKα) or HA-tagged IKKβ (HA-IKKβ). Lysates were checked for equal expression of transfected proteins by Western blotting (WB) with anti-HA antibodies. Labeled IKKγ mutants were added to lysates with either overexpressed HA-tagged IKKα or HA-tagged IKKβ and incubated for 30 min on ice. After immunoprecipitation (IP) with anti-HA antibodies, precipitated proteins were analyzed by SDS-PAGE and autoradiography. (B) (Left) In vitro-translated, [35S]methionine-labeled HA-tagged IKKβ, left untreated (−) or incubated with EGS (+), was analyzed for cross-linked IKKβ dimers after anti-HA immunoprecipitation (IP:HA), PAGE, and autoradiography. (Right) In vitro-translated, [35S]methionine-labeled, FLAG-tagged IKKγ1-196 was incubated without or with baculovirus-expressed, purified recombinant IKKβ and treated with EGS, as indicated. Cross-linked species were visualized after anti-FLAG immunoprecipitation (IP: FLAG) by PAGE and autoradiography. The positions of the monomeric and dimeric proteins are indicated by the arrows.