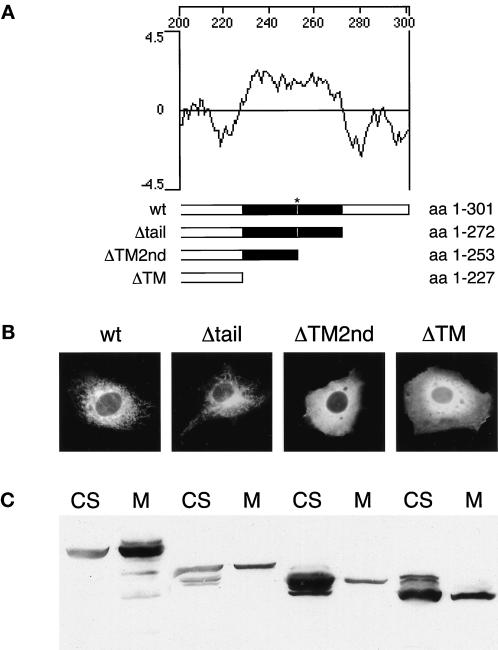
Figure 11.
Two carboxyl-terminal hydrophobic domains are required to anchor syntaxin 17 to membranes. (A) Kyte-Doolittle hydrophobicity plot of the carboxyl-terminal part of syntaxin 17 generated with the DNA-Star program. Shown below are the individual carboxyl-terminal deletion constructs. The putative transmembrane domains (in black) are separated by a single positively charged amino acid (K253, *). (B) NRK cells were transfected with amino-terminally myc-tagged syntaxin 17 full-length protein (amino acids [aa] 1–301; wt), myc–syntaxin 17 deleted of its carboxyl-terminal hydrophilic tail (aa 1–272; Δtail), myc–syntaxin 17 deleted of its carboxyl-terminal hydrophilic tail and its second hydrophobic domain (aa 1–253; ΔTM2nd), or myc–syntaxin 17 deleted of its carboxyl-terminal hydrophilic tail and both hydrophobic domains (aa 1–227; ΔTM). Cells were then fixed, permeabilized, and stained with anti-myc mAb. (C) COS-7 cells were transfected with the same syntaxin 17 deletion constructs as in B. Transfected cells were then fractionated into cytosolic (CS) and postnuclear membrane (M) fractions. Ten micrograms of protein was loaded onto each lane, separated by SDS-PAGE, and immunoblotted with anti-myc antibody. Note that the ratio of cytosolic to membrane-bound syntaxin17ΔTM2nd is dramatically increased compared with the full-length construct.