Abstract
OBJECTIVE
A previous study described the effect of a collaborative care intervention on improving adherence to antidepressant medications and depressive and functional outcomes of patients with persistent depressive symptoms 8 weeks after the primary care physician initiated treatment. This paper examined the 28-month effect of this intervention on adherence, depressive symptoms, functioning, and health care costs.
DESIGN
Randomized trial of stepped collaborative care intervention versus usual care.
SETTING
HMO in Seattle, Wash.
PATIENTS
Patients with major depression were stratified into severe and moderate depression groups prior to randomization.
INTERVENTIONS
A multifaceted intervention targeting patient, physician, and process of care, using collaborative management by a psychiatrist and a primary care physician.
MEASURES AND MAIN RESULTS
The collaborative care intervention was associated with continued improvement in depressive symptoms at 28 months in patients in the moderate-severity group (F1,87 = 8.65; P = .004), but not in patients in the high-severity group (F1,51 = 0.02; P = .88) Improvements in the intervention group in antidepressant adherence were found to occur for the first 6 months (χ2(1) = 8.23; P < .01) and second 6-month period (χ2(1) = 5.98; P < .05) after randomization in the high-severity group and for 6 months after randomization in the moderate-severity group(χ2(1) = 6.10; P < .05). There were no significant differences in total ambulatory costs between intervention and control patients over the 28-month period (F1,180 = 0.77; P = .40).
CONCLUSIONS
A collaborative care intervention was associated with sustained improvement in depressive outcomes without additional health care costs in approximately two thirds of primary care patients with persistent depressive symptoms.
Keywords: depression, primary care, collaborative care
Several studies have shown that only approximately 40% of primary care patients with major depression who are accurately diagnosed and started on antidepressant medication by their primary care physicians are significantly improved (50% decrease in initial symptoms) at 4 to 6 months.1–3 Recent evaluations of enhanced depression care interventions have shown significant improvement in the percent of patients who recover.1,2,4–6 We recently reported that a stepped collaborative care intervention resulted in significantly greater improvement in depressive and functional outcomes over a 6-month period compared to usual primary care in patients with persistent symptoms 2 months after initiating antidepressant medication treatment with their family physician.7,8
Because prior research had demonstrated that severity of depression was one of the best predictors of outcomes of depressive episodes in primary care,9 randomization of patients with persistent depression in the above trial was stratified by severity on the Hopkins Symptom Checklist depression items10 (symptom check list [SCL]-20 of <2.0 and >2.0). A second paper based on these 2 severity strata showed that severity of depression was a predictor of response to treatment.11 Analyses revealed a significant intervention effect by 3 months that continued at the 6-month follow-up among those with moderate depressive severity (mean SCL depression score of 1.0 to 2.0), but did not continue at the 6-month follow-up among those with greater symptom severity.11
The higher severity group represented about one third of the sample and was found to significantly differ from the moderate-severity group on 3 main clinical variables: the percent with comorbid panic disorder (odds ratio [OR], 5.8); percent reporting childhood emotional abuse (OR, 2.1); and percent feeling more lonely (OR, 2.6).11 Analysis of prescription refills for antidepressant medications over the 6-month period revealed improved adherence in intervention patients compared to controls in both high and moderate groups, suggesting that a worsening depressive course in the high-strata group between 3 and 6 months was not due to changes in medication adherence. These findings suggest that the higher depressive severity subgroup, characterized by increased loneliness and anxiety, was unlikely to show long-term benefits from the collaborative care intervention, whereas the moderate-severity group might show sustained benefits.
This paper will test several questions about the long-term effects of a stepped collaborative care intervention among persistently depressed primary care patients.
Among those in the moderate-severity strata, does the intervention (I) group sustain the 6-month improvement in depressive and functional outcomes over 28 months of follow-up as compared to usual-care (UC) patients?
Do the moderate- and high-severity intervention groups sustain the 6-month improvements in adequacy of dose and duration of antidepressant medication over 28 months of follow-up as compared to usual care?
What are the differences in total ambulatory care costs between intervention and control patients over this long-term follow-up?
METHODS
The settings for this study were 4 large primary care clinics of Group Health Cooperative of Puget Sound (GHC), an HMO serving over 400,000 persons in Western Washington. These 4 clinics, with a population of 88,000 enrollees, are staffed by 73 full- and part-time board-certified family physicians.
Eligibility for Randomization
Potentially eligible patients were identified using GHC automated registration, pharmacy, and visit data. Patients between the ages of 18 and 80 from 1 of the 4 primary care clinics who received a new antidepressant prescription (no prescriptions within the last 120 days) from a primary care physician for the diagnosis of depression or anxiety were eligible for the study. Five weeks after the prescription, patients received an approach letter from their primary care physicians inviting their participation in a study to improve the quality of care for various conditions.
At 8 weeks, patients received a call from the telephone survey team, who sought verbal informed consent for a 15-minute telephone screening interview to determine if they were eligible. The goal was to identify patients at high risk for persistent depression. The first stage screen included the telephone Structured Clinical Interview for Diagnostic and Statistical Manual IV (SCID).12 Criteria for selection for the second-stage interview were either having 4 or more residual major depressive symptoms or having recurrent depression (2 or more prior episodes) or dysthymia. The second-stage screen included the SCL-20 depressive items.10
Patients were excluded if they had a screening score of 2 or more on the CAGE alcohol screening questionnaire,13 were pregnant or currently nursing, planned to disenroll from the Group Health insurance plan within the next 12 months, were currently seeing a psychiatrist, had limited command of English, or had recently used lithium or antipsychotic medication.
Eligible and willing patients were informed that a research assistant would telephone them within the next week to arrange a second interview and explain the study in more detail. Inclusion criteria for the persistence study obtained during the first- and second-stage interviews included 1) 4 or more Diagnostic and Statistical Manual, Fourth Edition (DSM-IV) major depressive symptoms on the SCID, and a mean score of 1.0 or greater on the SCL-20 or 2) less than 4 DSM-IV major depressive symptoms but with a mean score of 1.5 or greater on the SCL-20. Patients were also screened for eligibility for a relapse prevention study that was run in parallel with the persistence study.14
The recruitment procedure and the study protocol were approved by the institutional review boards of the University of Washington and GHC. After a complete description of the study was given to the subjects, written informed consent was obtained. Consenting patients were randomly assigned to a collaborative model of care provided by both a psychiatrist and their primary care physician or to “usual care” provided by their primary care physician.
Usual Care
In most cases, usual care for depression provided by GHC family physicians involved prescription of an antidepressant medication, 2 or 3 visits over the first 6 months of treatment, and an option to refer to GHC mental health services. Both intervention and usual-care patients could also self-refer to a GHC mental health provider. GHC usually scores at about the seventy-fifth percentile on National Committee for Quality Assurance/Health Plan Employer Data and Information Set measures of quality of depression care.15
Stepped Collaborative Care Intervention
A multifaceted intervention was developed that targeted patients, physicians, and process of care. Each patient received a book16 and companion videotape17 developed by the study team, which reviewed the biopsychosocial model of depression, how medications and psychotherapy help depression, and how to become involved as an active partner with their physician in the care of their depressive illness. After the baseline interview and randomization, the research assistant scheduled 2 sessions for intervention patients with a psychiatrist (one 50-minute initial session and one 25-minute follow-up session) in the primary care clinic.
Intervention visits were usually spaced 2 weeks apart, with a brief telephone call to review progress between the first and second visits and, if necessary, between the third and fourth visits. The mean number of intervention visits was 2.75 ± 1.47 (range 0 to 7), and these were provided for a maximum of 3 months. The psychiatrist reviewed the course of the current depressive episode and the patient's biopsychosocial history. When severe side effects or inadequate response to treatment occurred, the psychiatrist helped the patient and primary care physician alter the dosage or choose an alternative medication. Intervention psychiatrists changed antidepressant medication due to side effects or nonresponse in 48% of patients, changed dosage of antidepressants in 64% of patients, augmented with a second antidepressant in 23% of patients, and referred to specialty mental health for additional psychotherapy in 12% of patients. Primary care physicians received immediate verbal consultation about their patient's progress and a typed psychiatric consultation note within 1 week.
Study Measures
Patients' adherence to antidepressant medication, severity of depressive symptoms, and functional impairment were assessed over a 28-month period after randomization by a telephone survey team blinded to the patients' randomization status.
Depressive Severity and Functional Impairment
The SCL-90 items that measure depression include 20 questions scored on a 0 to 4 measure of severity.10 We report the average item score of the 20 SCL items (range: 0 to 4.0). The SCL has been used in numerous studies of medical patients and has been found to have high reliability and validity.10 The SCL-20 depression items have also been shown to be more sensitive to change compared to several depression measures often used in psychiatric treatment trials in several large-scale primary care depression effectiveness trials.1–3
We used the SCID to diagnose current depression.12 A test–retest reliability study has found excellent agreement between in-person and telephone administration of the SCID.18
Seven items from the NEO neuroticism scale19 were used in this study because these items have been found to predict persistence of depressive symptoms in a primary care population, when controlling for depression severity.6
Functional impairment was measured with the Sheehan Disability Scale (SDS).20 The SDS contains 3 questions assessing interference in school and work, family, and home responsibilities with a score on each subscale and global score reported on a 0 to 10 Likert scale. It has been found in previous depression trials to have sensitivity to change over time.21
Medication Adherence to Adequate Dosage
Based on automated data from prescription refills, patients were rated on whether they received adequate dosage of antidepressant medication for 90 days or more during each of five 6-month blocks from randomization to 30 months. The lowest dosages in the ranges recommended in the Agency for Health Care Policy and Research guidelines22 and in guidelines developed for newer agents23 were used to define a minimum adequate dosage standard.
Comorbid Medical Conditions
Medical comorbidity was assessed using GHC's computerized prescription refill records. The Chronic Disease Score (CDS)-revised is a measure of chronic medical illness derived from the patient's use of prescription medications over a 6-month period.24 The CDS has been found to have high stability over a 1-year period and to prospectively predict as much of the variance in primary care visits, outpatients costs, and total costs as the ambulatory diagnostic groups (ADGs).25 It was also a better predictor of hospitalization and mortality than the ADGs.25
Health Care Costs
Health plan computerized data were used to identify all health services provided or paid for by GHC during the 6 months prior to randomization and in the 30 months after randomization (outpatient services for general medical care or for mental health and inpatient medical and mental health services). All GHC outpatient and inpatient services were assigned costs based on health plan accounting records (including actual personnel, supply, and overhead costs). Services purchased by GHC from external providers were assigned costs equal to the amount reimbursed by GHC. Visits to the consulting psychiatrist as part of the collaborative care intervention were assigned costs of $90 for each 50-minute visit and $55 for each 25-minute visit.
Randomization
Eligible patients with major depression were stratified into severe (SCL >2.0; n = 79) and moderate depression (SCL = 1.0 to 2.0; n = 149) groups based on their SCL-20 scores. Within each stratum, patients were randomized to the intervention or usual-care group in blocks of 8. Within each block, the randomization sequence was computer generated.
The study randomized 228 patients (intervention, n = 114; usual care, n = 114), who were included in the intent-to-treat 28-month analysis on depression and function. One hundred eighty-seven patients (82%) were enrolled at GHC for at least three of the five 6-month periods for at least 180 days per period. These patients had GHC automated data and were included in our cost and adherence analyses. The 41 patients who had disenrolled for three or more of the five 6-month periods were not included in the statistical analyses. In this sample of 187 patients, 119 had baseline SCL depression scores that placed them in the moderate-severity strata, and 68 had scores that placed them in the higher severity strata.
Statistical Analyses
t tests and χ2 analyses with corrections for continuity were used to examine differences between patients included in this study and those not included due to disenrollment. Descriptive differences between control and intervention patients were also tested using t tests for continuous variables and χ2 analyses with corrections for continuity for discrete data.
To determine if severity strata modified the effect of the treatment over the course of the study, we used random regression longitudinal modeling procedures.26 This longitudinal technique allows for the inclusion of data in the event of missing assessments as well as for random subject effects. The outcomes in these analyses were SCL depression, total Sheehan disability scores, and adherence to adequate dosage of medications. In these linear mixed models, we utilized main effect of time (1-, 3-, 6- and 28-month assessments), treatment group (intervention vs control), severity strata (moderate and severe), and covariates of baseline SCL depression level, age, gender, NEO neuroticism score, and CDS. In the analyses for the Sheehan disability score, baseline disability was also used as a covariate. To test the modification of the time × treatment interaction × severity strata, the 3-way interaction of strata × treatment × time was tested along with the three 2-way interactions. Since the purpose of this paper is to examine 28-month effects of the intervention, in the event of a significant 3-way interaction, planned post hoc tests were performed. These were analyses of covariance (ANCOVAs) on the 28-month outcomes separately for the 2 severity strata using treatment as the independent variable and the same covariates listed above. We did not test for intervention effects at the 1-, 3-, or 6-month assessments for the 2 strata, since that data has been presented elsewhere.11 For the adherence analyses, we used a dichotomous version of the random regression procedure with a logistic link.27 The time points for this analyses were five 6-month periods, with the outcome being adherent or not during that period. The design and the covariates were the same as those described above. Descriptive χ2 analyses with corrections for continuity were used to test intervention and control group differences in adherence to adequate dosage of medications within each severity strata.
Since the cost analyses require greater sample size to attain adequate power,28 and the observed mean costs for the 2 strata were very similar, we combined the strata for the cost analyses. The 4 major cost outcomes (outpatient depression treatment costs, nondepression outpatient costs, total ambulatory costs, and total health services costs) were tested for heteroscedasticity using F tests of variance. All measures showed homoscedasticity of variance. As a result, we used linear regression analyses with log-transformed data to test for intervention and control group differences in the 4 cost outcomes after adjusting for covariates.29 The covariates used were age, gender, CDS, and 6-month costs prior to baseline.
RESULTS
A total of 2,699 letters were mailed to eligible patients in the 4 Group Health primary care clinics, with 336 (12.4%) refusing to be interviewed and 312 (11.5%) patients who were ineligible or who couldn't be contacted. Patients agreeing to be interviewed (n = 2,051; 76.1%) did not differ in age or gender from patients refusing to be interviewed.
Of the 2,051 patients completing the screening, 272 (13.3%) were eligible for the persistence study and 694 (33.9%) were eligible for a parallel study on relapse prevention14 (based on having 0 to 3 DSM-IV symptoms and either recurrent depression or dysthymia). Of the 272 patients eligible for the persistence study, 176 (64.7%) received a baseline interview and 159 (90.3%) were successfully randomized to the persistence study. Ninety-six (35.3%) patients did not receive a baseline interview. Of these 96 patients, 30 refused, 30 did not have time to participate in an intervention program, 27 could not participate for other miscellaneous reasons, and 9 could not be contacted. Patients refusing baseline interview did not differ in age or gender from those agreeing to be interviewed.
Of the 694 patients eligible for the baseline interview for the relapse prevention study based on screening interview, 472 (68%) completed the interview and 69 (14.6%) were offered and accepted randomization to the persistence study based on an SCL of 1.5 or more. Patients refusing the baseline interview for the relapse prevention study (222 (8.1%)) did not differ in age or gender from those agreeing to be interviewed.
Of the 228 patients randomized, 209 (91.7%) completed the 1-month follow-up, 193 (84.6%) completed the 3-month follow-up, 192 (84.2%) completed the 6-month follow-up, and 171 (75%) completed the 28-month follow-up. There were no significant demographic or clinical differences between the patients who missed completing at least 1 follow-up and the patients completing all 4 follow-up interviews.
Patient Characteristics
There were no significant differences between the 114 intervention and 114 usual-care patients on the following demographic variables, including age (I, 47.2 ± 14.0 years vs UC, 46.7 ± 13.4 years), percent with 1 or more years of college (I, 77.2% vs UC, 78.1%), percent employed full- or part-time (I, 72.6% vs UC, 64.9%), and percent Caucasian (I, 79.8% vs UC, 80.7%). There was a significant difference between intervention and control patients in the percent of female subjects (I, 67.5% vs UC, 81.6%, χ2 (1) = 5.20; P = .02), which was one of the covariates controlled for when analyzing differences in outcomes between the 2 groups.
There were also no significant differences between intervention and control patients on chronic disease score (I, 1,191 ± 979 vs UC, 1,368 ± 1,293), NEO Neuroticism score (I, 22.7 ± 5.5 vs UC, 23.0 ± 5.5), SCL-Depression score (I, 1.9 ± 0.5 vs UC, 1.9 ± 0.5), percent with recurrent depression (I, 76.3% vs UC, 83.3%), or percent with a history of dysthymia (I, 50.0% vs UC, 59.8%).
There were no significant differences in age, gender, randomization or strata groups, or baseline depression level between the 187 patients used in the cost and adherence analyses and the 41 patients who disenrolled at some point during the 30 months. There were no significant demographic or clinical differences between control and intervention patients in the cost and adherence analyses.
Effects of Intervention on SCL Depression Score
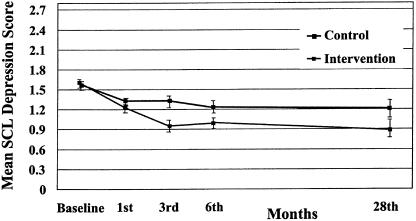
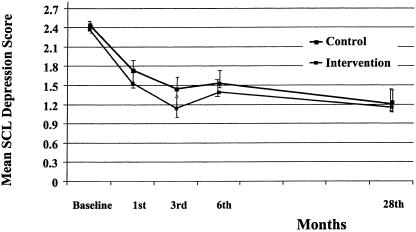
The longitudinal random regression model for SCL depression resulted in a significant 3-way interaction of time × treatment × strata (z = 2.16; P= .03). This was due to significant interactions of time × treatment (z = 1.94; P = .05) and time × strata (z = 3.16; P = .002). In this model, the main effects are not directly interpretable. Baseline depression, CDS, and NEO were significant covariates. On the basis of these results, we stratified by baseline severity in order to examine 28-month depression. The planned post hoc ANCOVAs for the 28-month SCL-Depression showed a significant treatment effect for patients in the moderate-severity strata (F1, 87 = 8.65; P = .004), with the adjusted mean for the control group (1.23 ± 0.62) significantly higher than that of the intervention group (0.88 ± 0.52) at the 28-month follow-up (seeFig. 1). In the high strata, treatment differences in SCL-Depression at 28 months were not statistically significant (F1, 51 = 0.02; P = .88] (seeFig. 2). The adjusted means were 1.16 ± 0.85 and 1.19 ± 0.72 for the intervention and control groups, respectively, at the 28-month follow-up.
FIGURE 1.
SCL depression adjusted means over time for moderate strata: intervention versus controls.
FIGURE 2.
SCL depression adjusted means over time for high strata: intervention versus controls.
Effects of Intervention on Sheehan Disability Scale
The longitudinal random regression model for Sheehan disability resulted in a significant 3-way interaction of time × treatment × strata (z = 3.08; P = .002). This was due to significant interactions of time × treatment (z = 2.06; P = .04) and time × strata (z = 3.69; P < .001), and treatment × strata (z = 2.41; P = .02). In this model, the main effects are not directly interpretable. CDS was the only significant covariate. On the basis of these results, we stratified by baseline severity in order to examine 28-month disability. The ANCOVAs for the Sheehan Disability Scale at the 28-month follow-up were not statistically significant. In the moderate strata, the control group reported more disability (adjusted mean = 3.58 ± 2.37) in comparison to the intervention group (adjusted mean = 3.09 ± 2.30) (F1, 87 = 1.21; P = .27). In the high strata, there were no significant differences between the intervention (adjusted mean = 3.41 ± 2.61) and control groups (adjusted mean = 3.20 ± 2.66) (F1, 51 = 0.09; P = .76).
Effects of Intervention on Dosage and Duration of Antidepressant Medications
The longitudinal ordinal random regression model examining patients meeting criteria for at least 90 or more days of an adequate dosage of antidepressants in each of five 6-month periods did not result in a significant 3-way interaction of time × treatment × strata (z = 1.79; P = .07). When this term was dropped, and the model was refit, the only significant 2-way interaction was time × treatment (z = 3.62; P < .001). In this model, the main effects of time and treatment are not directly interpretable. The strata main effect was very significant in the model (z = 4.49; P < .001). On the basis of this result, we stratified by baseline severity in order to examine 28-month adherence to an adequate dosage of medication. For the high-severity strata, intervention patients were significantly more likely to adhere to 90 days or more of antidepressants at the lowest dose consistent with Agency for Health Care Policy and Research guidelines compared to usual-care patients during the first and second 6-month blocks of time. In the high strata during the first 6 months, 72% (n = 24) of the intervention patients and 40% (n = 14) of the controls were adherent to an adequate dosage of medication (χ2 (1) = 8.23; P < .01). This trend was also seen in the second 6-month period: 70% (n = 23) of the intervention patients and 37% (n = 13) of the controls were adherent to an adequate dosage of medication (χ2(1) = 5.98; P < .05). For the moderate-severity strata, intervention patients were only more likely to adhere to 90 days or more of adequate dosage of antidepressants during the first 6-month block of time (76% of the intervention patients versus 46% of the controls, χ2(1) = 6.10; P < .05) Similar, but nonsignificant, trends were observed for the second 6-month block. For the other three 6-month periods, the percentages were very similar for the treatment groups in both strata.
Effects of Intervention on Costs
Table 1 describes the unadjusted difference in health care costs between intervention and control patients. On the basis of linear regression models, there were no significant differences between intervention and control patients in total ambulatory costs (F1,180 = 0.77; P = .40), total health care costs (F1,180 = 0.91; P = .34), depression treatment costs (F1,173 = 2.65; P = .10), or non–depression-related outpatient costs (F1,180 = 0.11; P = .74). In the descriptive unadjusted data, intervention patients were found to have nonsignificant trends toward higher outpatient depression costs over the 30-month period, whereas controls had nonsignificant trends toward higher total nondepression costs and total outpatient costs. The incremental depression costs in the intervention group were primarily due to the increased costs of longer term use of serotonin reuptake inhibitors.
Table 1.
| Controls (n = 92) | Intervention (n = 95) | |||
|---|---|---|---|---|
| Strata | Mean, $ | 95% CI, $ | Mean, $ | 95% CI, $ |
| Intervention groups | ||||
| Antidepressant prescriptions | 821 | 523 to 1120 | 1,398 | 940 to 1,856 |
| Specialty mental health visits | 616 | 353 to 880 | 469 | 273 to 666 |
| Primary care visits with mental health diagnosis | 317 | 238 to 394 | 265 | 205 to 326 |
| Intervention visits | 185 | 168 to 201 | ||
| Outpatient depression treatment costs | 1,754 | 1,289 to 2,220 | 2,317 | 1,710 to 2,924 |
| Primary care visits without mental health diagnosis | 1,617 | 1,340 to 1,893 | 1,341 | 1,109 to 1,572 |
| Diagnostic tests (lab and radiology) | 809 | 575 to 1,043 | 657 | 487 to 826 |
| Specialty medical visits | 1,272 | 909 to 1,635 | 1,230 | 918 to 1,544 |
| Emergency visits | 354 | 134 to 574 | 289 | 109 to 470 |
| Pharmacy not including antidepressants | 1,719 | 1,273 to 2,164 | 1,060 | 714 to 1,407 |
| Other outpatient costs | 999 | 573 to 1,424 | 892 | 448 to 1,336 |
| Total outpatient nondepression costs | 6,769 | 5,351 to 8,188 | 5,470 | 4,431 to 6,510 |
| Total outpatient costs | 8,524 | 5,059 to 9,989 | 7,787 | 6,595 to 8,980 |
| Inpatient care (medical + mental health) | 1,247 | 486 to 2,008 | 1,362 | 633 to 2,090 |
| Total health services costs | 9,799 | 7,763 to 11,834 | 9,192 | 7,504 to 10,880 |
Represents the subjects who were enrolled at least three of the five 6-month periods for at least 180 days.
Numbers range from 170 to 187 due to missing data.
All scores are annualized for missing cost periods.
Another cost of an intervention program for persistent depression would be the cost of instituting and maintaining a tracking system. We estimated that a program that would need to be developed by an HMO to screen patients 8 weeks after initiation of primary care physician antidepressant prescriptions would add approximately $67 per patient to the cost of the intervention.
DISCUSSION
This study demonstrated the long-term (28-month) effectiveness of a collaborative care intervention among patients with moderate depression severity. Most trials testing mental health or collaborative care interventions versus usual primary care in patients with major depression have found a significant effect in reducing depressive symptoms over 3 to 6 months compared to usual care that tended to decrease over the next half year.1,2,5,30 This is probably because major depression in most primary care patients is a relapsing-remitting illness with most episodes lasting less than 6 months.9 Thus, even depressed patients in usual primary care who are less likely to receive evidenced-based care tend to improve over a 6- to 12-month period. Our stepped-care program, however, selected patients with a higher risk of chronicity by requiring that patients have 4 or more DSM-IV symptoms approximately 8 weeks after initiation of antidepressant medication by the primary care physician. Our data from structured interviews showed that this methodology selected a persistently ill population; approximately 50% had double depression (dysthymia and major depressive episodes), and approximately 75% had experienced 3 or more major depressive episodes.7 A recent trial that showed long-term improvements over a 1-year period also selected patients for 2 years of high utilization and depression; these eligibility criteria also likely selected patients who had more persistent depressive symptoms.31 A second trial that showed longer term improvement in depressive symptoms over 12 to 18 months differed from the current trial in that patients were selected by screening for depression in primary care, with less than half of patients in the usual-care arm receiving depression-specific treatment.32
In the current study, the significant improvement in depressive symptoms in the moderate-strata intervention versus control group (with similar trends in function) persisted for 28 months despite the fact that intervention patients demonstrated significantly better adherence to adequate dosage of antidepressant medications for only the first 6 months. It may be that the more complete recovery of intervention patients in the first 6 months in the moderate-severity strata was associated with less risk of relapse over the 28-month period. Residual depressive symptoms have been found to be one of the most important risk factors for predicting relapse of depression in primary care patients with depression.33,34 Another factor associated with enhanced outcomes in intervention patients may have been the education and resulting activation regarding self-management of depression provided by the videotape, the book, and specialist counseling. In the high-severity strata, there were large differences in medication adherence for the first 12 months that then dissipated. In this more difficult to treat group, these large differences in medication adherence were inadequate to produce sustained improvements in depressive symptoms relative to usual care.
A strength of the study was the population-based provision of collaborative care to primary care patients who were persistently ill 8 weeks after initiation of pharmacologic treatment. Our previous collaborative care trials have shown that these interventions markedly improve outcomes for patients with major depression, but not minor depression.1,2 About half of patients diagnosed with depression in primary care and started on antidepressants have minor depression, and 65% to 70% of these patients recover over 1 to 3 months in usual primary care.1,2 Approximately 40% of patients with major depression also improve over 3 to 4 months in usual primary care. By closely monitoring 8-week outcomes of primary care treatment of depression, we targeted specialty mental health resources to the care of patients who had the most need for additional treatment. We found that approximately 22% of over 2,000 patients diagnosed and started on antidepressant medication by primary care physicians met our criteria for persistent symptoms, and most of the patients benefited from the short-term addition of specialty mental health care.7 Our results suggest that approximately one third of patients with persistent symptoms who have higher initial severity (and a high prevalence of comorbid anxiety with less social support)11 did not benefit from this brief intervention; these patients represent about 7% of patients diagnosed with depression and treated by primary care physicians.
The most conservative interpretation of the cost data suggests that for no greater total ambulatory costs, this stepped-care intervention was associated with improved outcomes in the moderate-severity-strata patients, who represent about two thirds of the total sample. In general, higher outpatient depression costs in intervention patients were balanced by higher outpatient nondepression costs in usual-care controls. This same pattern was seen in both intervention and control patients in both severity strata. For instance, in the moderate-severity strata, the total outpatient costs were $8,016 (95% confidence interval [95% CI], $6,568 to $9,464) in intervention patients and $8,824 (95% CI, $6,677 to $10,970) in usual-care patients; in the high-severity strata, the intervention patient total outpatient costs were $7,358 (95% CI, $5,159 to $9,556) versus $8,035 (95% CI, $6,293 to $9,778) in usual-care patients. This pattern of health care costs could be interpreted as evidence for a cost-offset effect (i.e., the increased resources provided for depression treatment lead to an off-setting reduction in general medical utilization). An important caution here is the lack of precision in estimates of total ambulatory costs. The 95% CIs increase progressively as one moves from estimates of depression costs to total ambulatory costs to total health care costs. The increase in depression costs in the intervention group was primarily due to better adherence to serotonin reuptake inhibitors. This increase in depression costs of approximately $500 based on pharmaceutical costs should decrease by 50% over the next 1 to 2 years due to increased use of generic serotonin reuptake inhibitors.
Several limitations in this study must be noted. First, the research was completed in 4 large HMO clinics staffed by family physicians, and implementing this intervention model may be more difficult in primary care networks that do not integrate psychiatric services into primary care. Second, the population was largely Caucasian, working, and middle class. The intervention may not generalize to other socioeconomic or ethnic groups. Third, this was a multifaceted intervention, and we are unable to state which components of the intervention were most important to its success. This limitation must be balanced by evidence that multifaceted interventions aimed at patient education and activation, physician education, and changing the process of care have been shown to be most effective in improving chronic disease management.35 Fourth, the study was underpowered to evaluate statistical differences in total ambulatory costs, which may require populations of over 1,000 patients given the large standard deviations in cost data.28 Finally, the CDS suggested more medical comorbidity in controls, which may have influenced the depression cost differences; however, our health care analyses controlled for CDS as well as for 6-month costs prior to randomization.
CONCLUSION
In summary, a stepped collaborative care intervention for primary care patients with persistent depression was associated with improved symptomatic outcomes for the approximately two thirds of patients who had moderate severity of depression over a 28-month period, with no evidence of an increase in total health care costs.
Acknowledgments
This work was supported by grants #MH-4-1739 and #MH 016473 from the National Institute of Mental Health Services Division in Bethesda, Md (Dr. Katon).
REFERENCES
- 1.Katon W, Von Korff M, Lin E, et al. Collaborative management to achieve treatment guidelines: impact on depression in primary care. JAMA. 1995;273:1026–31. [PubMed] [Google Scholar]
- 2.Katon W, Robinson P, Von Korff M, et al. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53:924–32. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 3.Simon G, Von Korff M, Heiligenstein J, et al. Fluoxetine versus tricyclic antidepressants: a randomized trial of effectiveness and cost in primary care. JAMA. 1996;275:1897–905. [PubMed] [Google Scholar]
- 4.Wells K, Sherbourne C, Schoenbaum M, et al. Impact of dissemination quality improvement programs for depression in managed primary care: a randomized controlled trial. JAMA. 2000;283:212–20. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- 5.Hunkeler E, Meresman J, Hargreaves W, et al. Efficacy of nurse telehealth care and peer support in augmenting treatment of depression in primary care. Arch Fam Med. 2000;9:700–8. doi: 10.1001/archfami.9.8.700. [DOI] [PubMed] [Google Scholar]
- 6.Simon G, Von Korff M, Rutter C, Wagner E. Randomized trial of monitoring, feedback and management of care by telephone to improve treatment of depression in primary care. BMJ. 2000;320:550–4. doi: 10.1136/bmj.320.7234.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trail. Arch Gen Psychiatry. 1999;56:1109–15. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 8.Lin E, Von Korff M, Russo J, et al. Can depression treatment in primary care reduce disability? A stepped care approach. Arch Fam Med. 2000;9:1052–58. doi: 10.1001/archfami.9.10.1052. [DOI] [PubMed] [Google Scholar]
- 9.Katon W, Lin E, Von Korff M, et al. The predictors of persistence of depression in primary care. J Affect Disord. 1994;31:81–90. doi: 10.1016/0165-0327(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 10.Derogatis L, Rickzels K, Uhlenhuth E, Covi L. The Hopkins Symptom Checklist: a measure of primary symptom dimensions. In: Pichot P, editor. Psychological Measurements in Psychopharmacology: Problems in Psychopharmacology. Basel: S. Karger AG; 1974. pp. 79–112. [Google Scholar]
- 11.Walker E, Katon W, Russo J, et al. Predictors of outcome in a primary care depression trial. J Gen Intern Med. 2000;15:859–67. doi: 10.1046/j.1525-1497.2000.91142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JB, Gibbon M, First MB, et al. The Structured Clinical Interview for DSM III-R (SCID): multi-site test-retest reliability. Arch Gen Psychiatry. 1992;49:630–6. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 13.Mayfield D, McLeod G, Hall P. The CAGE Questionnaire: validation of a new alcohol screening instrument. Am J Psychiatry. 1974;131:1121–3. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 14.Katon W, Rutter C, Ludman E, et al. A randomized trial of relapse prevention of depression in primary care. Arch Gen Psychiatry. 2001;58:241–7. doi: 10.1001/archpsyc.58.3.241. [DOI] [PubMed] [Google Scholar]
- 15.HEDIS. 1999 New Measure National Results: HEDIS users group monthly update. Washington DC: National Committee for Quality Assurance; 1999. [Google Scholar]
- 16.Katon W, Ludman E, Simon G, et al. The Depression Help Book. Boulder, Colo: Bull Publishing Company; 2002. [Google Scholar]
- 17.Katon W, Ludman E, Simon G, et al. Depression (recurrent and chronic): Self-Care Companion for Better Living. [videotape] New York: Time-Life Medical Patient Educational Media Inc; 1996. [Google Scholar]
- 18.Simon G, Revicki D, Von Korff M. Telephone assessment of depression severity. J Psychiatr Res. 1993;27:247–52. doi: 10.1016/0022-3956(93)90035-z. [DOI] [PubMed] [Google Scholar]
- 19.Costa P, McCrae R. The NEO Personality Inventory Manual. Odessa, Fla: Psychological Assessment Resources; 1985. [Google Scholar]
- 20.Sheehan DV, Harnett-Sheehan K, Raj B. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan M, Katon W, Russo J, Dobie R, Sakai C. A randomized trial of nortriptyline for severe chronic tinnitus: effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153:2251–9. [PubMed] [Google Scholar]
- 22.Agency for Health Care Policy and Research. Depression in Primary Care. Treatment of Major Depression. Vol. 2. Rockville, Md: U.S. Department of Health and Human Services; 1993. Publication AHCPR 93–0511. [Google Scholar]
- 23.Katzelnick D, Kobak K, Jefferson J, Greist J, Henk H. Prescribing patterns of antidepressant medications for depression in an HMO. Formulary. 1996;31:374–88. [Google Scholar]
- 24.Clark D, Von Korff M, Saunders K, Baluch W, Simon G. A chronic disease score with empirically derived weights. Med Care. 1995;33:783–95. doi: 10.1097/00005650-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29:452–72. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Hedeker D, Gibbons R. MIXREG: a computer program for mixed effects regression analysis with autocorrelated errors. Comput Methods Programs Biomed. 1996;49:229–52. doi: 10.1016/0169-2607(96)01723-3. [DOI] [PubMed] [Google Scholar]
- 27.Hedeker D, Gibbons R. MIXOR: a computer program for mixed effects ordinal regression analysis. Comput Methods Programs Biomed. 1996;49:157–76. doi: 10.1016/0169-2607(96)01720-8. [DOI] [PubMed] [Google Scholar]
- 28.Sturm R, Unützer J, Katon W. Effectiveness research and implications for study design: sample size and statistical power. Gen Hosp Psychiatry. 1999;21:274–83. doi: 10.1016/s0163-8343(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 29.Diehr P, Yanez D, Ash A, Hornbrook M, Lin D. Methods for analyzing health care utilization and cots. Annu Rev Public Health. 1999;20:125–44. doi: 10.1146/annurev.publhealth.20.1.125. [DOI] [PubMed] [Google Scholar]
- 30.Lin E, Simon G, Katon W, et al. Can enhanced acute-phase treatment of depression improve long-term outcomes. A report of randomized trials in primary care. Am J Psychiatry. 1999;156:643–5. doi: 10.1176/ajp.156.4.643. [DOI] [PubMed] [Google Scholar]
- 31.Katzelnick D, Simon G, Pearson S, et al. Randomized trial of a depression management program in high utilizers of medical care. Arch Fam Med. 2000;9:345–51. doi: 10.1001/archfami.9.4.345. [DOI] [PubMed] [Google Scholar]
- 32.Unützer J, Rubenstein L, Katon W, et al. Two-year effects of quality improvement on medication management for depression. Arch Gen Psychiatry. 2001;58:935–42. doi: 10.1001/archpsyc.58.10.935. [DOI] [PubMed] [Google Scholar]
- 33.Lin E, Katon W, Von Korff M, et al. Relapse of depression in primary care. Rate and clinical predictors. Arch Fam Med. 1998;7:443–9. doi: 10.1001/archfami.7.5.443. [DOI] [PubMed] [Google Scholar]
- 34.Simon G. Long-term prognosis of depression in primary care. Bull World Health Organ. 2000;78:439–44. [PMC free article] [PubMed] [Google Scholar]
- 35.Katon W, Von Korff M, Lin E, Simon G. Rethinking practitioner roles in chronic illness: the specialist, primary care physician and practice nurse. Gen Hosp Psychiatry. 2001;23:138–44. doi: 10.1016/s0163-8343(01)00136-0. [DOI] [PubMed] [Google Scholar]