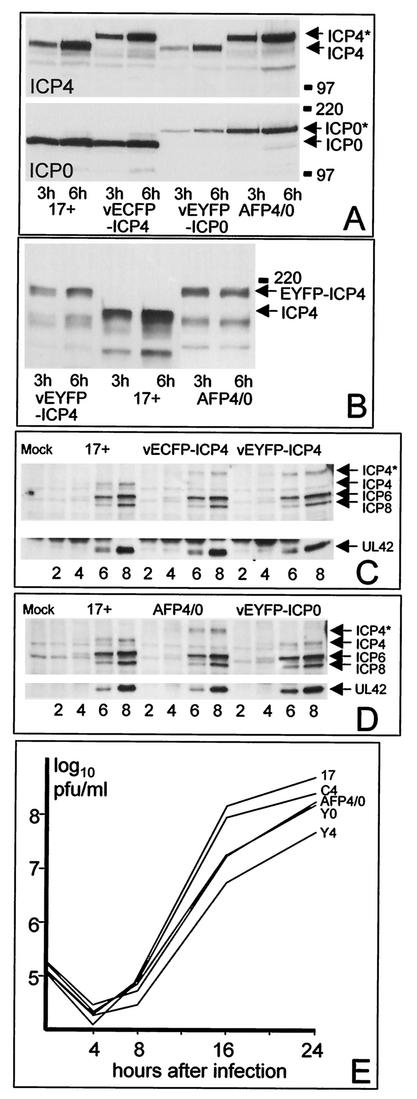
FIG. 1.
(A) BHK cells were infected with the viruses 17+, vECFP-ICP4, vEYFP-ICP4, and AFP4/0 (MOI = 10) as indicated, and whole-cell extracts were prepared 3 or 6 h after virus adsorption. The proteins were separated on 7.5% polyacrylamide gels, transferred to a nitrocellulose filter, and then detected by probing the same filter for ICP4 and ICP0 sequentially. The gel mobilities of the normal proteins are represented by the 17+ samples; the recombinant viruses express either the normal protein or the autofluorescent fusion protein of lower mobility, as expected from their genotypes. The positions of selected molecular mass markers (in kilodaltons) and the ICP4 and ICP0 proteins are indicated. The asterisks indicate the AFP versions of ICP4 and ICP0. (B) The same experiment as in panel A was conducted with viruses vEYFP-ICP4, 17+, and AFP4/0 probed for expression of ICP4. (C) Vero cells were infected with 17+, vECFP-ICP4, and vEYFP-ICP4 (MOI = 2), and whole-cell extracts were prepared 2, 4, 6, and 8 h after adsorption. The samples were analyzed by Western blotting for the expression of ICP4, ICP6, ICP8, and UL42. ICP4* represents the autofluorescent version of the protein. (D) The same experiment as in panel C was conducted using viruses 17+, AFP4/0, and vEYFP-ICP0. (E) Growth curves of the autofluorescent viruses. BHK cells in parallel 35-mm-diameter plates were infected with the indicated viruses at a multiplicity of 0.5 PFU per cell. The cells and medium were harvested 4, 8, 16, and 24 h after virus adsorption, and the samples were titrated along with the input aliquots on Vero cells. The data are derived from a single experiment but are representative of several similar experiments.