Abstract
The genome analysis of Kaposi's sarcoma-associated herpesvirus (KSHV) has revealed the presence of an open reading frame (ORF 4) with sequence homology to complement control proteins. To assign a function to this protein, we have now expressed this ORF using the Pichia expression system and shown that the purified protein inhibited human complement-mediated lysis of erythrocytes, blocked cell surface deposition of C3b (the proteolytically activated form of C3), and served as a cofactor for factor I-mediated inactivation of complement proteins C3b and C4b (the subunits of C3 convertases). Thus, our data indicate that this KSHV inhibitor of complement activation (kaposica) provides a mechanism by which KSHV can subvert complement attack by the host.
The complement system is a first line of immune defense against all foreign pathogens, including viruses. It serves as both an innate and an acquired immune defense against viruses (16). However, during the course of several million years of coevolution with their hosts, certain viruses have developed defenses that involve immune evasion strategies targeting the complement system. Important examples of such viruses include the poxviruses, herpesviruses, retroviruses, paramyxoviruses, and picornaviruses (1, 2, 9, 20, 22, 23).
Kaposi's sarcoma-associated herpesvirus (KSHV), or human herpesvirus 8 (HHV-8), a likely etiologic agent of Kaposi's sarcoma, was the first gammaherpesvirus to be identified in humans since the discovery of Epstein-Barr virus. It is present in nearly all epidemiologic forms of Kaposi's sarcoma and has now also been linked with pleural effusion lymphomas and multicentric Castleman's disease (11). The genome sequencing of KSHV has revealed the presence of an open reading frame (ORF 4) that is structurally similar to a family of human complement control proteins (CCPs) known as regulators of complement activation (RCA) (14). Such CCP homologs (CCPHs) have also been found in poxviruses (7, 13, 15), herpesvirus saimiri (3), and murine gammaherpesvirus 68 (5).
The RCA proteins are characterized by the presence of common structural motifs, the short consensus repeat (SCR) or CCP modules. Each SCR is comprised of approximately 60 to 70 amino acids and is characterized by a conserved motif that includes four disulfide-linked cysteines, prolines, tryptophan, and many other hydrophobic residues, which together form a bead-like structure (8). The RCA family includes the plasma proteins factor H and C4bp and the membrane proteins CR1 (CD35), CR2 (CD21), DAF (CD55), and MCP (CD46). These proteins, with the exception of CR2, act at the level of C3 and C4 and function by dissociating the subunits of C3 and C5 convertases and/or by acting as cofactors for the factor I-dependent cleavage of C3b and/or C4b (17).
The KSHV ORF 4 is a 1,650-nucleotide ORF that encodes a protein with four SCRs. As a result of alternative splicing, the ORF 4 protein is thought to be expressed both as a membrane-bound 550-amino-acid protein with a putative transmembrane region at amino acids 517 to 545 and as a shorter secreted form that lacks the transmembrane region (11, 14). The sequence similarity between the ORF 4 protein and the human CCPs CR1, CR2, DAF, MCP, factor H, and C4bp varies from 44 to 55%. Although the ORF 4 protein is composed of tandemly repeating SCR domains with significant sequence similarity to human CCPs, this similarity by itself is not a sufficient criterion for assigning complement-inhibitory function to this protein. For example, human CR2 and vaccinia virus protein B5R are composed of SCRs, but they do not inhibit complement activity (8, 24).
To determine whether the ORF 4 protein actually possesses complement-inhibitory activity, we have expressed the soluble form of this protein (SCRs 1 to 4 without the transmembrane region) using the Pichia expression system. In particular, in designing the construct, we did not include the His tag that is normally added during protein expression to aid in purification, since addition of His residues might introduce a stretch of charge on the protein (if the pKa of His is altered upon binding) and thus influence its activity.
In brief, the ORF 4 protein encompassing SCRs 1 to 4 was amplified from the HHV-8 clone CB43 (a kind gift of Jens-Christian Albrecht and Frank Neipel, Institut für Klinische und Molekulare Virologie, Erlangen, Germany), with specific primers 5′-GGAATTCAAGTGTTCCCAAAAAACCTTAATTGG-3′ (EcoRI site is underlined) and 5′-GCTCTAGATTACAAAACACACTTAGGAAGTGG-3′ (XbaI site is underlined, and stop codon is in boldface), and cloned into the yeast expression vector pPICZα at EcoRI and XbaI sites. After the authenticity of the clone was confirmed by sequencing the DNA, it was integrated into the Pichia genome according to the manufacturer's instructions (Pichia expression kit, version F; Invitrogen, Carlsbad, Calif.).
The expressed protein was concentrated from the culture supernatant by ammonium sulfate precipitation (80%) and purified by using a series of chromatographic procedures, which involved separation of the protein on a DEAE-Sephacel column (Sigma, St. Louis, Mo.) in 10 mM sodium phosphate (pH 7.4), containing 250 mM NaCl, followed by a heparin-agarose column (Sigma) in 10 mM sodium phosphate (pH 7.4) and a Mono S column (Pharmacia, Uppsala, Sweden) in 5 mM sodium acetate (pH 4.0). Elution of the expressed protein from these columns was achieved with a linear salt gradient of 0 to 1 M. Our purification data clearly indicated that, like the vaccinia virus CCP (VCP) (21), the ORF 4 protein, with its five putative heparin-binding sites, also bound to heparin (data not shown). Among the human complement regulators, factor H and C4bp bind to heparin (18), and interaction of factor H with heparin is an important step in the regulation of activation of the alternative pathway (12). Whether the interaction of the ORF 4 protein with heparin is fortuitous or has any physiological relevance requires further study.
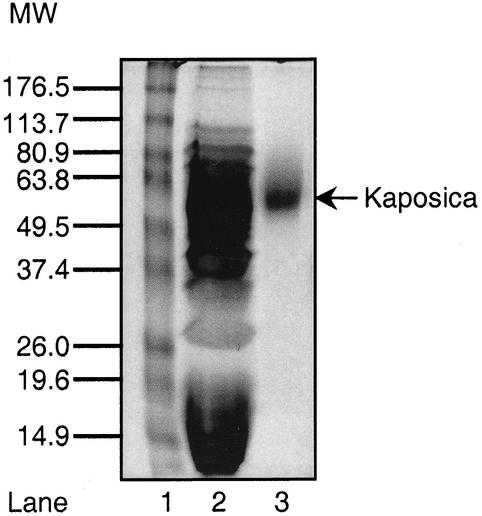
The purified ORF 4 protein migrated as a single band of 56,000 Da (Fig. 1). To confirm the identity of this protein, it was subjected to automated Edman degradation (Biomolecular Research Facility, University of Virginia, Charlottesville). The predicted N-terminal sequence of this protein is E-A-E-A-E-F-K-C-S-Q-K-T-L, where the first six residues, EAEAEF, are the result of cloning the ORF 4 protein into the Pichia vector. The amino acid sequence of the expressed protein corresponded to the predicted sequence, thus confirming its identity and purity and indicating that the signal peptide was cleaved in the mature protein. The calculated mass of the expressed protein was 27,838 Da, but it migrated as a band of 56,000 Da. This apparent higher molecular mass could reflect glycosylation of the protein, since the primary sequence contains three potential N-linked and several potential O-linked carbohydrate sites.
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the purified ORF 4 protein (kaposica). Culture supernatant from an ORF 4 protein-expressing clone and the purified ORF 4 protein were run on a sodium dodecyl sulfate-10% polyacrylamide gel under reducing conditions and stained with Coomassie blue. Lane 1, molecular weight (MW) markers (numbers in kilodaltons); lane 2, culture supernatant; lane 3, purified kaposica.
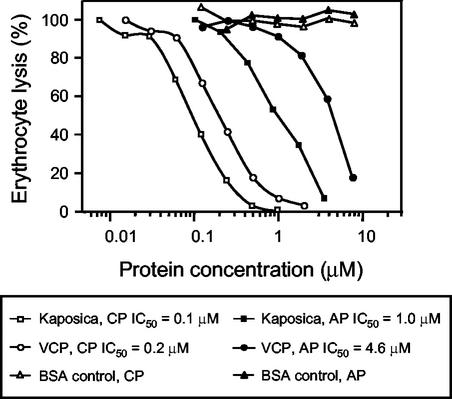
To assign a function to the ORF 4 protein, we studied the ability of the expressed protein to inhibit the complement-mediated lysis of erythrocytes (19), comparing its activity to that of VCP, which was expressed and purified as previously described (15). We chose hemolytic assays because these assays have been used in the past to quantitate the effect of CCPs and inhibitors (6, 15); thus, 50% inhibitory concentrations would be useful for comparison purposes. We found that the HHV-8 ORF 4 protein was approximately five times more potent than VCP in inhibiting the alternative pathway-mediated lysis of rabbit erythrocytes and twice as effective in inhibiting the classical pathway-mediated lysis of sheep erythrocytes (Fig. 2). Thus, our data clearly demonstrate that the ORF 4 encodes a functional RCA that is capable of inhibiting both the classical and alternative pathways of complement activation.
FIG. 2.
Inhibition of alternative and classical pathway-mediated lysis of erythrocytes by kaposica and VCP. The relative effects of kaposica and VCP on the alternative pathway (AP)-mediated lysis of rabbit erythrocytes and the classical pathway (CP)-mediated lysis of antibody-coated sheep erythrocytes were examined as previously described (19). Bovine serum albumin (BSA) was included as a nonspecific control. IC50, 50% inhibitory concentration.
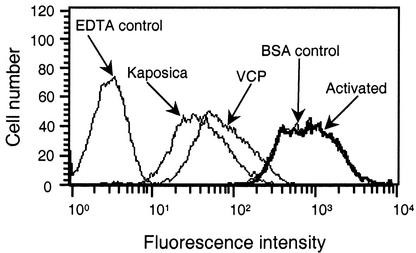
Activation of either the classical or the alternative pathway leads to the formation of C3 convertases (C4b,2a and C3b,Bb), which in turn cleave C3 to generate C3a and C3b. The newly formed C3b attaches covalently to the activating surface, which then furthers the activation process by initiating the formation of the membrane attack complex (8). The RCA proteins are known to act at the level of the C3 convertase, and previous studies have shown that viral homologs of CCP also regulate C3 convertase formation (3, 5, 10, 13, 15). The data depicted in Fig. 3 indicate that the ORF 4 protein inhibits the deposition of C3b on the activating surface. This effect of the ORF 4 protein is specific, as bovine serum albumin (a nonspecific control) had no effect on the C3b deposition. Thus, like other viral homologs, the ORF 4 protein apparently acts at the level of C3 convertase formation.
FIG. 3.
Inhibition of C3b deposition on erythrocytes during complement activation by kaposica and VCP. Classical pathway-mediated C3b deposition on erythrocytes was measured by incubating 5 μl of antibody-coated sheep erythrocytes (109/ml in GVB++: 5 mM barbital, 145 mM NaCl, 0.5 mM MgCl2, 0.15 mM CaCl2, and 0.1% gelatin, pH 7.4) with 1 μl of C8-deficient human serum (Calbiochem, San Diego, Calif.) and 44 μl of GVB++ or GVB++ containing 2 μM kaposica or VCP at 37°C for 30 min. Deposition of C3b was detected by fluorescence-activated cell sorting with fluorescein isothiocyanate-conjugated F(ab′)2 anti-C3 goat immunoglobulin G (Cappel Laboratories, Warrington, Pa.). Control samples contained either 10 mM EDTA or 2 μM bovine serum albumin (BSA).
The RCA proteins regulate C3 convertase formation either by dissociating the C3 convertases into their subunits (C4b,2a into C4b and 2a, and C3b,Bb into C3b and Bb) or by serving as a cofactor for factor I-dependent cleavage of C3b and C4b (the subunits of C3 convertases). Dissociation of C3 convertase limits complement activation; however, the dissociated subunits of C3 convertases (C3b and C4b) are capable of forming new C3 convertases. In contrast, factor I-mediated cleavage of C3b and C4b leads to the formation of inactive subunits that are unable to participate in the formation of C3 convertase.
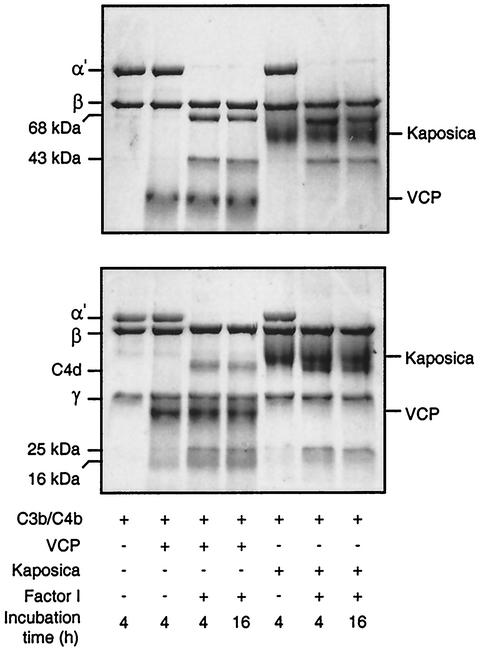
In order to further define the mechanism of C3 convertase regulation by the ORF 4 protein, we asked whether it is capable of serving as a cofactor for factor I-mediated cleavage of C3b and C4b. The data provided in Fig. 4 show that incubation of C3b or C4b with the ORF 4 protein and factor I results in cleavage of C3b and C4b; incubation of C3b or C4b with factor I alone did not result in C3b or C4b cleavage (data not shown). Thus, our data indicate that the ORF 4 protein supports the factor I-mediated cleavage of both C3b and C4b. These results suggest that the ORF 4 protein effectively regulates C3 convertase formation by converting C3 convertase subunits into their inactive form, which is no longer able to participate in the formation of C3 convertases. This property is known to be shared by only two other viral CCPHs, VCP (15) and SPICE (13).
FIG. 4.
Analysis of factor I cofactor activity of kaposica and VCP for complement proteins C3b (upper panel) and C4b (lower panel). Cofactor activity was observed by incubating C3b or C4b (4 μg) with kaposica (2 μg) or VCP (2 μg) and factor I (100 ng) in 15 μl of 10 mM sodium phosphate, pH 7.4, containing 145 mM NaCl at 37°C for the indicated time period. Cleavage products were visualized by running the samples on sodium dodecyl sulfate-8 and 9% polyacrylamide gels for C3b and C4b, respectively, and staining them with Coomassie blue. During C3b cleavage, the α′ chain is cleaved into N-terminal 68-kDa and C-terminal 43-kDa fragments; the appearance of these fragments indicates the generation of iC3b (inactive C3b). In the case of C4b cleavage, the α′ chain is cleaved into N-terminal 25-kDa, C-terminal 16-kDa, and central C4d fragments; these cleavages result in inactivation of C4b and generation of C4c and C4d.
In summary, our data clearly show that the HHV-8 ORF 4 protein encodes a functional regulator of human complement. Based on these activities, we have named the protein kaposica (the KSHV inhibitor of complement activation). A recent in vivo study of the gammaherpesvirus 68 virus, a murine gammaherpesvirus, has shown that the gammaherpesvirus 68 RCA protein serves as a virulence determinant of the gammaherpesvirus 68 (4). Based on our data and the recent report on gammaherpesvirus 68, we would like to propose that kaposica may be involved in protecting KSHV from the host's complement system and is probably required for its successful in vivo survival and effective spread.
Acknowledgments
We thank Jens-Christian Albrecht and Frank Neipel (Institut für Klinische und Molekulare Virologie, Erlangen, Germany) for providing the HHV-8 CCPH clone, Maria Rosa Sarrias (Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia) for expanding the HHV-8 CCPH clone, and Michael Pangburn (Department of Biochemistry, University of Texas Health Science Center, Tyler) for the generous gift of complement proteins C3 and factor I. We also express appreciation to Yogesh Panse and Sharanabasava for excellent technical assistance and Ashwini Atre for fluorescence-activated cell sorting analysis.
This work was supported by the Wellcome Trust Overseas Senior Research Fellowship in Biomedical Science in India to A.S. and National Institutes of Health grants AI30040 and AI048487 to J.D.L. We also acknowledge financial assistance to A.K.S. by the Council of Scientific and Industrial Research, India.
REFERENCES
- 1.Bernet, J., J. Mullick, A. K. Singh, and A. Sahu. Viral mimicry of the complement system. J. Biosci., in press. [DOI] [PMC free article] [PubMed]
- 2.Cooper, N. R. 1998. Complement and viruses, p. 393-407. In J. E. Volanakis and M. M. Frank (ed.), The human complement system in health and disease. Marcel Dekker, Inc., New York, N.Y.
- 3.Fodor, W. L., S. A. Rollins, S. Bianco-Caron, R. P. Rother, E. R. Guilmette, W. V. Burton, J. C. Albrecht, B. Fleckenstein, and S. P. Squinto. 1995. The complement control protein homolog of herpesvirus saimiri regulates serum complement by inhibiting C3 convertase activity. J. Virol. 69:3889-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapadia, S., B. Levine, S. Speck, and H. Virgin. 2002. Critical role of complement and viral evasion of complement in acute, persistent, and latent gamma-herpesvirus infection. Immunity 17:143-155. [DOI] [PubMed] [Google Scholar]
- 5.Kapadia, S. B., H. Molina, V. van Berkel, S. H. Speck, and H. W. Virgin IV. 1999. Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J. Virol. 73:7658-7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostavasil, I., A. Sahu, H. M. Friedman, R. J. Eisenberg, G. H. Cohen, and J. D. Lambris. 1997. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J. Immunol. 158:1763-1771. [PubMed] [Google Scholar]
- 7.Kotwal, G. J., S. N. Isaacs, R. Mckenzie, M. M. Frank, and B. Moss. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250:827-830. [DOI] [PubMed] [Google Scholar]
- 8.Lambris, J. D., A. Sahu, and R. Wetsel. 1998. The chemistry and biology of C3, C4, and C5, p. 83-118. In J. E. Volanakis and M. Frank (ed.), The human complement system in health and disease. Marcel Dekker, Inc., New York, N.Y.
- 9.Lubinski, J., T. Nagashunmugam, and H. M. Friedman. 1998. Viral interference with antibody and complement. Semin. Cell Dev. Biol. 9:329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mckenzie, R., G. J. Kotwal, B. Moss, C. H. Hammer, and M. M. Frank. 1992. Regulation of complement activity by vaccinia virus complement-control protein. J. Infect. Dis. 166:1245-1250. [DOI] [PubMed] [Google Scholar]
- 11.Means, R. E., S. M. Lang, Y. H. Chung, and J. U. Jung. 2002. Kaposi's sarcoma associated herpesvirus immune evasion strategies. Front. Biosci. 7:e185-e203. [DOI] [PubMed]
- 12.Meri, S., and M. K. Pangburn. 1990. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc. Natl. Acad. Sci. USA 87:3982-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosengard, A. M., Y. Liu, Z. Nie, and R. Jimenez. 2002. Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc. Natl. Acad. Sci. USA 99:8808-8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahu, A., S. N. Isaacs, A. M. Soulika, and J. D. Lambris. 1998. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J. Immunol. 160:5596-5604. [PubMed] [Google Scholar]
- 16.Sahu, A., and J. D. Lambris. 2001. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 180:35-48. [DOI] [PubMed] [Google Scholar]
- 17.Sahu, A., D. Morikis, and J. D. Lambris. 2000. Complement inhibitors targeting C3, C4, and C5, p. 75-112. In J. D. Lambris and V. M. Holers (ed.), Therapeutic interventions in the complement system. Humana Press Inc., Totowa, N.J.
- 18.Sahu, A., and M. K. Pangburn. 1993. Identification of multiple sites of interaction between heparin and the complement system. Mol. Immunol. 30:679-684. [DOI] [PubMed] [Google Scholar]
- 19.Sahu, A., and M. K. Pangburn. 1996. Investigation of mechanism-based inhibitors of complement targeting the activated thioester of human C3. Biochem. Pharmacol. 51:797-804. [DOI] [PubMed] [Google Scholar]
- 20.Smith, G. L., J. A. Symons, A. Khanna, A. Vanderplasschen, and A. Alcami. 1997. Vaccinia virus immune evasion. Immunol. Rev. 159:137-154. [DOI] [PubMed] [Google Scholar]
- 21.Smith, S. A., N. P. Mullin, J. Parkinson, S. N. Shchelkunov, A. V. Totmenin, V. N. Loparev, R. Srisatjaluk, D. N. Reynolds, K. L. Keeling, D. E. Justus, P. N. Barlow, and G. J. Kotwal. 2000. Conserved surface-exposed K/R-X-K/R motifs and net positive charge on poxvirus complement control proteins serve as putative heparin binding sites and contribute to inhibition of molecular interactions with human endothelial cells: a novel mechanism for evasion of host defense. J. Virol. 74:5659-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spear, G. T., M. Hart, G. G. Olinger, F. B. Hashemi, and M. Saifuddin. 2001. The role of the complement system in virus infections. Curr. Top. Microbiol. Immunol. 260:229-245. [DOI] [PubMed] [Google Scholar]
- 23.Stoiber, H., A. Clivio, and M. P. Dierich. 1997. Role of complement in HIV infection. Annu. Rev. Immunol. 15:649-674. [DOI] [PubMed] [Google Scholar]
- 24.Vanderplasschen, A., E. Mathew, M. Hollinshead, R. B. Sim, and G. L. Smith. 1998. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc. Natl. Acad. Sci. USA 95:7544-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]