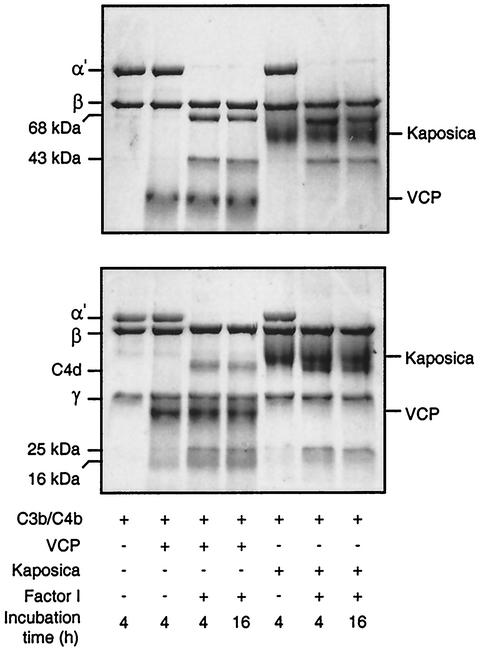
FIG. 4.
Analysis of factor I cofactor activity of kaposica and VCP for complement proteins C3b (upper panel) and C4b (lower panel). Cofactor activity was observed by incubating C3b or C4b (4 μg) with kaposica (2 μg) or VCP (2 μg) and factor I (100 ng) in 15 μl of 10 mM sodium phosphate, pH 7.4, containing 145 mM NaCl at 37°C for the indicated time period. Cleavage products were visualized by running the samples on sodium dodecyl sulfate-8 and 9% polyacrylamide gels for C3b and C4b, respectively, and staining them with Coomassie blue. During C3b cleavage, the α′ chain is cleaved into N-terminal 68-kDa and C-terminal 43-kDa fragments; the appearance of these fragments indicates the generation of iC3b (inactive C3b). In the case of C4b cleavage, the α′ chain is cleaved into N-terminal 25-kDa, C-terminal 16-kDa, and central C4d fragments; these cleavages result in inactivation of C4b and generation of C4c and C4d.