Abstract
OBJECTIVE
To examine the impact of immediate concurrent feedback on dose adjustment in patients with renal failure.
DESIGN
Prospective 12-month study in patients with various degrees of renal failure, with comparison to a retrospective control group.
SETTING
A 39-bed unit of a university hospital providing primary and tertiary care.
PATIENTS
Patients with renal failure (estimated creatinine clearance ≤50 mL/min) receiving at least 1 pharmacologically active drug.
INTERVENTIONS
Education of physicians and immediate concurrent feedback on the ward giving estimated creatinine clearance and dose recommendations for renally eliminated drugs adjusted to individual renal function.
MEASUREMENTS AND MAIN RESULTS
The percentage of dosage regimens adjusted to renal function and cost assessment of drug therapy were calculated. Overall, 17% of the patients had at least 1 estimated creatinine clearance ≤50 mL/min. In the intervention group, the dose of 81% of renally eliminated drugs was adjusted to renal function, compared with 33% in the control group ( P < .001). The mean difference in cost between standard and adjusted dose of renally eliminated drugs in the intervention and control groups was 5.3 ± 12.3 and 0.75 ± 2.8 Swiss francs (approximately US$3.5 and US$0.5), respectively ( P < .001), accounting for 16.5% and 2.8%, respectively, of daily medication costs of all drugs.
CONCLUSIONS
The proportion of doses of renally eliminated drugs adjusted to renal function can be substantially increased by immediate concurrent feedback. This saves drug costs and has the potential to prevent adverse drug reactions.
Keywords: kidney failure, drug therapy, drug costs
The adjustment of drug dosage to individual patient requirements can maximize therapeutic efficacy and minimize adverse drug reactions. Dose adjustment in hepatic or renal failure is especially critical because parent compounds or active metabolites can accumulate and cause additional morbidity and costs .1,2 The average adverse drug event occurring in a hospital increases the length of stay by 2 days and generates costs of approximately US$2,400.3,4
Many drugs (e.g., aminoglycoside antibiotics, H 2-receptor antagonists, angiotension-converting enzyme [ACE] inhibitors, digoxin, and lithium) are eliminated primarily unchanged through the kidney.5 Dose adjustment in renal failure can easily be accomplished by estimating the creatinine clearance based on serum creatinine, age, weight, and gender6–8; calculating the individual elimination capacity for a given drug; and adjusting dose and/or dosing interval.7,8 Dose adjustment in patients with renal failure reduces both the costs of drug therapy and the risk of adverse drug reactions.9,10 The need for appropriate dose reductions in patients with renal failure is illustrated by earlier studies in which ranitidine-associated central nervous system (CNS) adverse drug reactions occurred more frequently in patients with renal impairment11 and the incidence of imipenem/cilastatin-associated seizures was reduced after dosage adjustment to renal function.12
Several strategies have been used aiming to change the way physicians prescribe drugs, e.g., education, preparation of guidelines, or checking drug orders by the hospital pharmacy. All are effective if patient-specific reminders are given at the time of consultation.13,14 For example, antibiotic and antiulcer drug prescribing was successfully modified by immediate concurrent feedback, i.e., by real-time application of specific and explicit recommendations.15,16
The aims of the present study were to assess the incidence of inappropriate dosing of renally excreted drugs in hospitalized patients with renal failure and to test the impact of immediate concurrent feedback by pharmacist participation on clinical rounds on dose adjustment of these drugs and on medication costs.
METHODS
Setting
The study was conducted at the General Internal Medicine Service of the University Hospital of Basel, an 870-bed teaching hospital providing primary and tertiary care to an urban population of approximately 200,000 inhabitants and tertiary care to northwest Switzerland. The wards consisted of 3 adjacent 13-bed units located on the same floor specialized in the care of patients with infectious diseases, kidney disorders including post-transplantation care, and oncological diseases. Each unit was staffed with 1 medical intern in his second or third year of postgraduate training on a 6-month rotation. The 3 interns were supervised by 2 full-time chief residents board-certified in internal medicine. Before this study, no structured system for dose optimization in patients with renal failure was in place, with the exception of a successful dose optimization program for aminoglycosides, which was not included in the evaluation.
Definitions and Calculations
Two primary outcomes were calculated: (1) percentage of dosage regimens adjusted to renal function and (2) cost assessment of drug therapy.
Creatinine clearance (mL/min) was estimated for all patients according to the following equation:
where k is 0.9 for women and 1.1 for men, and 1 mg/dL = 88 μmol/L serum creatinine.7,8 Whenever serum creatinine concentration was determined, creatinine clearance was estimated. Creatinine clearance was estimated at least once in all patients; in the majority of patients, it was estimated repeatedly. Renal failure was defined as creatinine clearance ≤50 mL/min and was subdivided into 3 categories: mild (31–50 mL/min), moderate (10–30 mL/min), and severe (<10 mL/min). Severe renal failure often required dialysis. The rationale for selecting the threshold of 50 mL/min was that the relationship between creatinine clearance and plasma concentration (or half-life or area under the curve) of a drug eliminated predominantly by the kidneys is exponential, plasma concentration increases markedly if creatinine clearance falls below 50 mL/min, and dosage should be adjusted.7
For all administered drugs the fraction of the bioavailable dose which is eliminated extrarenally (Qo) was extracted from the literature; for prodrugs, the Qo value of the active drug was used.17 If at least 70% of the bioavailable, active drug was eliminated by the kidney as unchanged compound (Qo ≤ 0.3), dose adjustment according to the patient's individual renal function was considered mandatory in patients with renal failure (see discussion for explanation of chosen threshold). The individual elimination capacity (Q) was calculated as follows7,8:
Doses were then adjusted using 1 of the following procedures:
adjusted dose = standard dose × Q (standard dose interval unchanged)
adjusted dose interval = standard dose interval/Q (standard dose unchanged)
combination of (1) and (2) in order to obtain a practicable dosage scheme.
Standard dose and dose interval were defined as those required for the treatment of the corresponding disease in a patient with normal renal function as approved by the Swiss health authorities and published in the product information.18 Since most orally administered drugs are available only in a small number of dosages and because some drugs cannot be divided (e.g., coated formulations), precise dose adjustment was not possible in all cases. In these instances, a dose modification was recommended which reduced the dose as much as possible, but was never below the calculated dose for the actual degree of renal impairment. The prescribed drug regimen was always adjusted to the most recent estimated creatinine clearance. In case a follow-up creatinine was determined, creatinine clearance was estimated, and dose requirements were calculated accordingly.
Cost calculations were based on public prices of the smallest package size. For drugs with at least 70% of the bioavailable, active drug eliminated by the kidney as unchanged compound (Qo ≤ 0.3), potential savings resulting from dose adjustment in patients with renal failure were evaluated using the following equation:
Patients
Consecutive patients were evaluated who were admitted to 1 of the units of the General Internal Medicine Service and were prescribed at least 1 pharmacologically active drug. Those who had at least 1 estimated clearance value of 50 mL/min or less were included in the study. The intervention group consisted of all eligible patients admitted within a 12-month period in 1995 and 1996. For the control group, data for all patients admitted to the same ward in 1993 were evaluated retrospectively, and a random sample of 50% of all patients with renal failure was selected. The following parameters were collected for all patients with renal failure: age, gender, serum creatinine, weight, length of hospital stay, International Classification of Diseases, Ninth Revision diagnostic codes, estimated creatinine clearance, and the doses of all administered drugs.
Intervention
The intervention was performed by a clinical pharmacist (ADF), who had graduated from the School of Pharmacy of Basel and was a full-time member of the staff of the hospital's Division of Clinical Pharmacology and the Institute of Hospital Pharmacy. At the beginning of the intervention and after each rotation of interns, the physicians were informed about design and goals of the study during a half-hour oral presentation by the clinical pharmacist, followed by a 7-week period with weekly half-hour oral presentations to familiarize them with the essential dosing rules. There was no financial or other incentive offered to the staff for making dose adjustments. The clinical pharmacist alternately joined the regular rounds of 1 unit on weekdays, thus seeing each patient every fourth day. The participation on ward rounds was a means to integrate the pharmacist into the team and to search for patients with renal failure by reviewing the charts. The charts of the patients of the remaining 2 units were reviewed daily by the clinical pharmacist after her ward round. Whenever serum creatinine was determined for a patient, the pharmacist calculated the patient's creatinine clearance and optimized dosages for all drugs with Qo values of 0.3 or less. For all drug regimens which were not appropriately adjusted to renal function, optimized dosages were proposed to the responsible physicians directly at the ward round or within 24 hours (on weekends within 48 hours) after the serum creatinine measurement for those units where the clinical pharmacist did not participate at the regular round.
To test whether explicit dose recommendations are necessary or whether the knowledge of reduced individual renal function was sufficient to prompt the expected dose adjustment, the intervention was separated in 2 steps during a 4-month period. First, the clinical pharmacist only calculated the estimated creatinine clearance and informed the responsible physician by writing a note. Then, 24 hours later, drug dosing was checked in the patient's chart. If the dose was still too high, an explicit dose adjustment was recommended to the prescribing physician in a second step.
Statistics
Results are expressed as mean values ±1 SD, proportions, or medians, as appropriate. For 2-sample comparisons of continuous variables that were normally distributed, the t test, and for continuous variables that were not normally distributed, the Mann-Whitney rank sum test was used. Categorical comparisons were made using the χ2statistic with the Yates correction to reduce the risk of false-positive conclusions. A 2-sided P≤.05 was considered statistically significant. All tests were performed with the software package Sigmastat (Jandel Scientific GmbH, Erkrath, Germany).
Estimation of Risk of Adverse Drug Reactions
The relative risk of adverse drug reactions (RRADR) in patients receiving dosages not adjusted to renal function can be defined as follows:
Consecutively, reduction of RRADRby the intervention can be calculated as
where ncis the number of patients in the control group receiving drugs of interest in adjusted or excessive dosages and niis the number of the respective patients in the intervention group receiving drugs of interest. In patients receiving adjusted doses, the RRADRis 1 and, thus, does not appear in the equation.
RESULTS
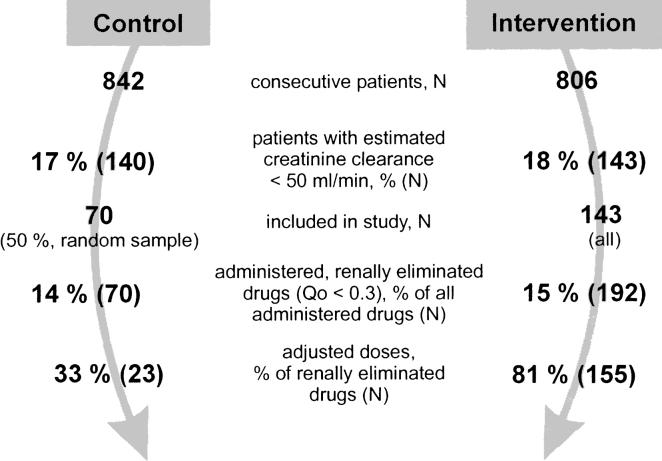
A total of 1,648 patients were evaluated (Figure 1). About 1 of every 6 patients evaluated (283, 17%) had at least 1 estimated creatinine clearance of 50 mL/min or less and was eligible for the study. Patients with renal failure in the intervention ( N =143) and control group ( N =70) were comparable with respect to gender, length of hospital stay, and the severity of renal failure. The patients in the control group, however, were significantly older and received fewer drugs (Table 1). One seventh of the drugs prescribed (15%) were eliminated with at least 70% of the drug unchanged renally (i.e., Qo ≤ 0.3). On average, each patient with renal failure (creatinine clearance ≤50 mL/min) received about 1 renally eliminated drug (Qo ≤ 0.3).
FIGURE 1.
Design and main results of study in which drug dosage was adjusted to renal failure using an immediate concurrent feedback strategy.
Table 1.
Demographic Data
| Parameter | Control Group | Intervention Group | P-Value |
|---|---|---|---|
| N | 70 | 143 | — |
| Age, mean ± SD (median), y | 75.7 ± 13.9 (76.0) | 68.8 ± 17.6 (74.0) | <.005 |
| Male, n(%) | 37 (53) | 73 (51) | NS |
| Length of stay, mean ± SD (median), d | 23.1 ± 25.8 (17.5) | 20.9 ± 16.0 (16.0) | NS |
| Creatinine clearance, mean ± SD (median), mL/min | 26.0 ± 14.2 (24.5) | 23.9 ± 14.1 (23.0) | NS |
| Severe renal failure, n(%) | 12 (17) | 35 (25) | — |
| Moderate renal failure, n(%) | 31 (44) | 55 (38) | — |
| Mild renal failure, n (%) | 27 (39) | 53 (37) | — |
| Drugs prescribed per patient, mean ± SD (median), n | 7.0 ± 3.6 (6.0) | 8.9 ± 4.1 (9.0) | <.005 |
| Drugs prescribed with Qo ≤0.3 per patient, mean ± SD (median), n | 1.0 ± 0.8 (1.0) | 1.4 ± 1.3 (1.0) | NS |
Qo indicates the fraction of active drug eliminated nonrenally.
In the control group, doses were adjusted to individual renal function in only 33% of renally eliminated drugs (Qo ≤ 0.3) (Table 2). In contrast, in the intervention group, 81% of all doses which required adjustment were correct ( P < .001 vs control). In patients receiving doses that had not been adjusted to the degree of renal failure, the dose was always too high-never too low. Drugs often prescribed in doses which were too high included digoxin, amoxicillin, ciprofloxacin, flucloxacillin, norfloxacin, atenolol, sotalol, enalapril, ranitidine, fluconazole, and acyclovir.
Table 2.
Drug Dosage
| Control group | Intervention group | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Total, N | Correct, n(%) | Excessive, n(%) | Total, N | Correct, n(%) | Excessive, n(%) | P-Value |
| No. of drugs | 70 | 23 (33) | 47 (67) | 192 | 155 (81) | 37 (19) | <.001 |
| Digoxin | 18 | 16 (89) | 2 (11) | 37 | 32 (86) | 5 (14) | NS |
| Antibiotics* | 25 | 4 (16) | 21 (84) | 54 | 42 (78) | 12 (22) | <.001 |
| Cardiovascular drugs | 4 | 1 (25) | 3 (75) | 39 | 30 (77) | 9 (23) | NS |
| H2-blocking agents | 20 | 1 (5) | 19 (95) | 51 | 41 (80) | 10 (20) | <.001 |
| Antimycotics | 3 | 1 (33) | 2 (67) | 8 | 8 (100) | 0 (0) | NS |
| Antivirals | 0 | 0 | 0 | 3 | 2 (67) | 1 (33) | — |
Aminoglycosides for which an effective therapeutic drug monitoring program was in place were not included.
In order to evaluate patient characteristics associated with the lack of adherence to the dose recommendations, patients receiving excessive doses in the intervention and control group were pooled and compared with those receiving adjusted doses. Patients with dosages which were too high had a significantly higher estimated creatinine clearance than those receiving appropriate doses (mean, 30 ± 14 mL/min; median, 32, for those receiving excessive doses vs mean, 22 ± 14 mL/min, median, 18, for those receiving appropriate doses; P <.001). There was no difference in the duration of hospital stay between the 2 groups (mean, 22 ± 17 days; median, 18, for those receiving excessive doses vs mean, 21 ± 21 days; median, 15, for those receiving adjusted doses, NS).
During a 4-month period, the intervention was separated into 2 steps. First, the physician was informed of the estimated creatinine clearances. Second, explicit dose recommendations were given 24 hours later if dosage was not adjusted to renal function. In only 8 (31%) of the 26 patients with renal failure receiving renally eliminated drugs (Qo ≤ 0.3) was the dose adjusted to individual renal function after the first step. Of the remaining 18 patients, the dose was adjusted in 11 patients after the explicit dose recommendation was communicated to the physician, resulting in 19 (73%) of the 26 patients receiving appropriate doses.
In patients with renal failure, the costs of renally eliminated drugs after dose adjustment were compared to the costs of the standard (i.e., unadjusted daily) dose (Table 3). The difference between standard and actually administered dose in the control and intervention group was 0.75 Swiss francs and 5.3 Swiss francs, respectively (approximately US$ 0.5 and US$ 3.5, P < .001), which amounted to 2.8% and 16.5% of the cost of all (i.e., renally and nonrenally eliminated) drugs which were administered to these patients.
Table 3.
Drug Costs*
| Parameter | Control Group | Intervention Group | P Value |
|---|---|---|---|
| Daily medication costs of all drugs per patient, mean ± SD (median) | 26.5 ± 38.8 (10.9) | 26.9 ± 27.6 (18.4) | NS |
| Daily medication costs of drugs with Qo†≤0.3 per patient, mean ± SD (median) | 5.9 ± 9.9 (2.0) | 5.0 ± 10.0 (1.6) | NS |
| Daily savings‡ of drugs with Qo ≤0.3 per patient, mean ± SD (median) | 0.75 ± 2.8 (0.0§) | 5.3 ± 12.3 (1.1) | <.001 |
| Daily savings§ of drugs with Qo ≤0.3 per patient, % of daily medication costs of all drugs before adjustment | 2.8 | 16.5 |
All drug costs are Swiss francs. 1 Swiss franc ≈ US $0.66.
Qo is the fraction of the bioavailable dose which is eliminated extrarenally.
Savings is the cost of standard (i.e., unadjusted) dose − cost of actually administered dose.
The dose was not adjusted and the difference between standard and administered was 0 in most patients; the median is 0.
Approximately 4 hours per day were necessary for monitoring the 39 patients in the study ward. This included calculation of creatinine clearance and participation at the rounds of 1 unit (2 hours) during which all currently administered drug doses were checked. In addition, all prescriptions of the 2 other units were evaluated daily by chart review, and dose recommendations were calculated, if necessary; each required an additional hour.
The risk of CNS adverse drug reactions was estimated for ranitidine, the drug most frequently prescribed in the study population using the data published by Slugg et al.11 Assuming that the risk of CNS adverse drug reactions in patients with renal impairment and receiving adjusted doses is comparable to the risk in patients with normal renal function, RR ADRis 4.50, and the reduction of RR ADRby the intervention is 2.64. This suggests that the risk of CNS adverse drug reactions in patients receiving ranitidine, which was the case in a third (71 of 213) of all our patients, was reduced by about 60%.
DISCUSSION
Strategies to adjust drug dosage in renal failure may help to individualize drug therapy and, thus, contribute to drug safety. Decreases of renal function may profoundly affect drug disposition through changes in protein binding and drug excretion. Among the most common causes of renal dysfunction in hospitalized patients is increasing age. However, moderate degrees of renal failure are often overlooked because serum creatinine concentrations closely reflect renal function only after adjustment for age, gender, and weight. In this study, 1 in 6 patients had renal insufficiency to a degree that should prompt physicians to substantial dose modifications in order to avoid a marked increase in steady state concentrations of drugs that are eliminated predominantly by the kidneys. This percentage (17%) is in contrast with 2 other studies where the incidence was 5%19 and 40%.20 The low percentage found by Cantu et al.,19 compared with our population, can be explained by the lower cutoff of creatinine clearance (<40 mL/min) and the lower age (48 ± 18 years in patients without renal failure and 64 ± 15 years in patients with renal failure). In the study of Vogt and Dayer,20 more patients with primary kidney disorders might have been included.
On average, each patient with renal failure received 1 drug eliminated primarily by the kidney (Qo ≤ 0.3, see Table 4). For drugs with Qo values of 0.3 (i.e., 70% is eliminated renally in active form), the dosage has to be reduced by a third in patients with a creatinine clearance of 50 mL/min and halved in those with a clearance of 25 mL/min in order to avoid drug accumulation. Thus, for drugs with a Qo of 0.3 or less, dosage can usually be adjusted easily and in a practicable way by reducing the dose and/or increasing the dosing interval. However, it has to be emphasized that even drugs with higher Qo values may exhibit a clinically relevant elevation of plasma concentration in patients with renal failure. For example, the concentration of drugs with Qo values of 0.5 (e.g., pindolol, pravastatin5) is increased by about 70% in patients with a creatinine clearance of 20 mL/min, compared with patients with normal creatinine clearance values. In addition, drugs which are eliminated predominantly by metabolism (i.e., with a high Qo value) may have active or toxic metabolites that accumulate in renal failure, as is the case for pethidine,21 morphine,22 and midazolam.23 Hence, when these cases are addressed in a future intervention, dose adjustment in renal failure will become even more important.
Table 4.
Drugs with a Qo value ≤0.3 (eliminated extrarenally) and Whose Dosage Has to Be Adjusted in Renal Failure*
| Drug | Qo-Value |
|---|---|
| Digoxin | 0.30 |
| Lithium | 0.02 |
| Aminoglycosides and vancomycin | |
| Amikacin | 0.02 |
| Gentamicin | 0.02 |
| Netilmicin | 0.01 |
| Tobramycin | 0.02 |
| Vancomycin | 0.05 |
| β-Lactam antibiotics | |
| Ampicillin | 0.06 |
| Cefaclor | 0.25 |
| Cefalexin | 0.04 |
| Flucloxacillin | 0.30 |
| Virostatics | |
| Acyclovir | 0.10 |
| Cidofovir | 0.13 |
| Famciclovir | 0.14 |
| Ganciclovir | 0.05 |
| Lamivudine | 0.03 |
| Valaciclovir | 0.10 |
| Zalcitabine | 0.30 |
| H2-receptor antagonists | |
| Cimetidine | 0.30 |
| Famotidine | 0.20 |
| Nizatidine | 0.30 |
| Ranitidine | 0.20 |
| ACE inhibitors | |
| Cilazapril(at) | 0.20 |
| Enalapril(at) | 0.10 |
| Quinapril(at) | 0.20 |
| Fibrates | |
| Bezafibrate | 0.15 |
| Clofibrate | 0.10 |
| Fenofibrate | 0.20 |
| β-Blockers | |
| Atenolol | 0.12 |
| Carteolol | 0.30 |
| Sotalol | 0.15 |
| Nadolol | 0.25 |
| Antiepileptic | |
| Gabapentin | 0.02 |
| Vigabatrin | 0.01 |
The Qo value is the fraction of the bioavailable dose that is eliminated extrarenally. A Qo value of 0.30 means that 70% of the bioavailable dose is excreted unchanged in the urine.
In the current study, after explicit dosage recommendations of the clinical pharmacist, 81% of dosages were correct, compared with 33% in the control group. This compares favorably with 3 other studies where pharmacists of the hospital pharmacy reviewed drug orders, contacted the physician if necessary, and made a dose recommendation for renally eliminated drugs. The recommendation was accepted in 75%10,24 and 88%9 of cases. Nevertheless, in our intervention group, the dosage of 19% of renally eliminated drugs was not adjusted to renal failure, i.e., was too high. The reasons might be as follows:
It may not always be correct to adjust the dosage regimen exclusively based on pharmacokinetic considerations. For example, ACE inhibitors (e.g., enalapril) or β-blocking agents (e.g., atenolol) with large pharmacodynamic variability should be dosed according to clinical end points such as blood pressure or heart rate.
In patients with variable renal function, rapidly changing serum creatinine values may not permit appropriate estimation of renal function.
Cost as the only motivation for dose adjustment may not be considered sufficient by the physician for drugs with a wide therapeutic window (e.g., ranitidine).
Although one might expect less adverse drug reactions to occur with correct dosage, adjusting the dose of renally eliminated drugs was not associated with a shorter hospital stay. However, our power to detect such a difference was limited. An earlier study in our institution revealed that about 4% of all patients encounter severe adverse drug reactions, most of which were dose-dependent,25 suggesting that they may require transfer to the intensive care unit. The equation to calculate length of stay in the present study was not designed to detect these cases because only patient-days spent on the general ward were counted.
If only the estimated creatinine clearance was communicated to the physician without making exact dosage recommendations, doses were not more frequently adjusted to renal function in the intervention compared with the control group. Thus, the pertinent element of the intervention was the immediate feedback process giving explicit dosage recommendations. This may not indicate that the concurrent training program for interns was useless since it may have increased the acceptance of dose recommendations given by the pharmacist. However, other studies have shown that education alone is usually not successful in changing prescribing behavior13 or physician awareness for drug safety issues.25
In the present study, the time point of intervention was the earliest possible (i.e., the prescription process, when all the pertinent information for deciding on the optimal dosage is available). This has the advantage of putting the pharmacist in an active role, and is considered to be much more effective than the reactive role of responding to prescription errors long after the decision has been made.26
The dose modifications implemented by the intervention resulted in a net cost reduction of 4.55 Swiss francs (approximately US$3) of daily medication costs for patients with renal impairment. Taking into account the proportion of patients with renal failure (17%), this translates to a mean saving of 0.77 Swiss francs (approximately US$0.5) per patient per day. Considering the reduced drug prices offered to hospitals, about 250,000 patient-days are needed for the yearly salary of 1 pharmacist. This roughly corresponds to the number of patient-days per year of the University Hospital of Basel. However, in order to adjust the dosage of all patients (870 beds) as performed in this study, about 10 full-time pharmacists would be needed, which would be unjustifiably expensive. Computer-assisted monitoring of drug dosing may be a promising way to reach all patients when doses are prescribed and before inappropriate doses are administered. Others have successfully used computer-assisted dosing recommendations in patients with renal failure. However, these projects were limited to a small number of drugs,24,27 the intervention was not performed at the earliest time point possible (i.e., the prescription process), and the concept of calculating individual elimination capacity was not thoroughly applied.9,10,24,27
Because the system in place in the hospital did not offer the possibility of making an overall cost analysis of care per patient (e.g., flat rates per day were calculated irrespective of the intensity of care necessary for the individual patient), we had to restrict the cost analysis to cost of drugs. This neglects the potential influence of excessive dosing on management of adverse events and is therefore likely to underestimate the impact of such an intervention.
The results of this study indicate that in a considerable number of patients, the presence of renal dysfunction is not considered in drug prescription, resulting in excessive and avoidable costs and in potential risk for adverse drug reactions. This study clearly documented that an immediate concurrent feedback strategy implemented directly on the ward may result in a substantial reduction of inappropriate drug regimens, which is likely to translate into risk reductions for concentration-dependent (avoidable) adverse drug reactions. Our concept of dose adaptation can easily be used for a broad range of drugs and the ease of dose adaptation bears promising potential for integration in an electronic expert system used at the time of prescribing. As a first step, we have developed an application calculating dosage recommendations after entering the pertinent patient parameters (http://www.dosing.de).
Acknowledgments
This study was supported in part by grants from the Senglet Stiftung Basel, the Fonds Golaz of the Schweizerische Apothekerverein Bern-Liebefeld, and the Freiwillige Akademische Gesellschaft Basel; a grant from Mr. and Mrs. Wilhelm V.T. Martius-Fasser; a grant of the Wissenschaftliche Kredit of the University Hospital Basel; and BMBF grant 01EC9902.
REFERENCES
- 1.Jick H. Adverse drug effects in relation to renal function. Am J Med. 1977;62:514–7. doi: 10.1016/0002-9343(77)90406-5. [DOI] [PubMed] [Google Scholar]
- 2.Verbeeck RK, Branch RA, Wilkinson GR. Drug metabolites in renal failure: pharmacokinetic and clinical implications. Clin Pharmacokinet. 1981;6:329–45. doi: 10.2165/00003088-198106050-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277:307–11. [PubMed] [Google Scholar]
- 4.Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301–6. [PubMed] [Google Scholar]
- 5.Taeschner W, Vozeh S. Pharmacokinetic drug data. In: Speight TM, Holford NHG, editors. Avery's Drug Treatment. 4th ed. Auckland: Adis International; 1997. pp. 1629–64. [Google Scholar]
- 6.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 7. Quadri L. Arzneimitteldosierung bei Niereninsuffizienz. Analyse eines Modells. Inauguraldissertation. Universität Basel.
- 8.Dettli L. The kidney in pre-clinical and clinical pharmacokinetics. Jpn J Clin Pharmacol Ther. 1984;15:241–54. [Google Scholar]
- 9.Peterson JP, Colucci VJ, Schiff SE. Using serum creatinine concentrations to screen for inappropriate dosage of renally eliminated drugs. Am J Hosp Pharm. 1991;48:1962–4. [PubMed] [Google Scholar]
- 10.Goldberg DE, Baardsgaard G, Johnson MT, Jolowsky CM, Shepherd M, Peterson CD. Computer-based program for identifying medication orders requiring dosage modification based on renal function. Am J Hosp Pharm. 1991;48:1965–9. [PubMed] [Google Scholar]
- 11.Slugg PH, Haug MT, Pippenger CE. Ranitidine pharmacokinetics and adverse central nervous system reactions. Arch Intern Med. 1992;152:2325–9. [PubMed] [Google Scholar]
- 12.Pestotnik SL, Classen DC, Evans RS, Stevens LE, Burke JP. Prospective surveillance of imipenem/cilastatin use and associated seizures using a hospital information system. Ann Pharmacother. 1993;27:497–501. doi: 10.1177/106002809302700418. [DOI] [PubMed] [Google Scholar]
- 13.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342:1317–22. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 14.Greco PJ, Eisenberg JM. Changing physicians' practices. N Engl J Med. 1993;329:1271–3. doi: 10.1056/NEJM199310213291714. [DOI] [PubMed] [Google Scholar]
- 15.Seto WH, Ching T-Y, Kou M, Chiang S-C, Lauder IJ, Kumana CR. Hospital antibiotic prescribing successfully modified by “immediate concurrent feedback”. Br J Clin Pharmacol. 1996;41:229–34. doi: 10.1111/j.1365-2125.1996.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumana CR, Ching TY, Cheung E, et al. Antiulcer drug prescribing in hospital successfully influenced by “immediate concurrent feedback”. Clin Pharmacol Ther. 1998;64:569–74. doi: 10.1016/S0009-9236(98)90141-2. [DOI] [PubMed] [Google Scholar]
- 17.Dettli L, et al. Pharmakokinetische Daten für die Dosisanpassung. In: Biollaz J, Dayer P, Galeazzi RL, et al., editors. Grundlagen der Arzneimitteltherapie. 13th ed. Basel: Documed; 1993. pp. 13–21. [Google Scholar]
- 18.Morant J, Ruppanner H. Arzneimittelkompendium der Schweiz. Basel: Documed; 1995. [Google Scholar]
- 19.Cantu TG, Ellerbeck EF, Yun SW, Castine SD, Kornhauser DM. Drug prescribing for patients with changing renal function. Am J Hosp Pharm. 1992;49:2944–8. [PubMed] [Google Scholar]
- 20.Vogt N, Dayer P. Clinical pharmacology in practice [in French] Schweiz Med Wochenschr. 1994;124:2096–9. [PubMed] [Google Scholar]
- 21.Szeto HH, Inturrisi CE, Houde R, Saal S, Cheigh J, Reidenberg MM. Accumulation of normeperidine, an active metabolite of meperidine, in patients with renal failure of cancer. Ann Intern Med. 1977;86:738–41. doi: 10.7326/0003-4819-86-6-738. [DOI] [PubMed] [Google Scholar]
- 22.Osborne RJ, Joel SP, Slevin ML. Morphine intoxication in renal failure: the role of morphine-6-glucuronide. Br Med J (Clin Res Ed) 1986;292:1548–9. doi: 10.1136/bmj.292.6535.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer TM, Ritz R, Haberthür C, et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet. 1995;346:145–7. doi: 10.1016/s0140-6736(95)91209-6. [DOI] [PubMed] [Google Scholar]
- 24.McMullin ST, Reichley RM, Kahn MG, Dunagan C, Bailey TC. Automated system for identifying potential dosage problems at a large university hospital. Am J Health Syst Pharm. 1997;54:545–9. doi: 10.1093/ajhp/54.5.545. [DOI] [PubMed] [Google Scholar]
- 25.Schlienger RG, Luscher TF, Schoenenberger RA, Haefeli WE. Academic detailing improves identification and reporting of adverse drug events. Pharm World Sci. 1999;21:110–5. doi: 10.1023/a:1008631926100. [DOI] [PubMed] [Google Scholar]
- 26.Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267–70. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- 27.Preston SL, Briceland LL, Lomaestro BM, Lesar TS, Bailie GR, Drusano GL. Dosing adjustment of 10 antimicrobials for patients with renal impairment. Ann Pharmacother. 1995;29:1202–7. doi: 10.1177/106002809502901202. [DOI] [PubMed] [Google Scholar]