Abstract
OBJECTIVE
To establish rates of and risk factors for cardiac complications after noncardiac surgery in veterans.
DESIGN
Prospective cohort study.
SETTING
A large urban veterans affairs hospital.
PARTICIPANTS
One thousand patients with known or suspected cardiac problems undergoing 1,121 noncardiac procedures.
MEASUREMENTS
Patients were assessed preoperatively for important clinical variables. Postoperative evaluation was done by an assessor blinded to preoperative status with a daily physical examination, electrocardiogram, and creatine kinase with MB fraction until postoperative day 6, day of discharge, death, or reoperation (whichever occurred earliest). Serial electrocardiograms, enzymes, and chest radiographs were obtained as indicated. Severe cardiac complications included cardiac death, cardiac arrest, myocardial infarction, ventricular tachycardia, and fibrillation and pulmonary edema. Serious cardiac complications included the above, heart failure, and unstable angina.
MAIN RESULTS
Severe and serious complications were seen in 24% and 32% of aortic, 8.3% and 10% of carotid, 11.8% and 14.7% of peripheral vascular, 9.0% and 13.1% of intraabdominal/intrathoracic, 2.9% and 3.3% of intermediate-risk (head and neck and major orthopedic procedures), and 0.27% and 1.1% of low-risk procedures respectively. The five associated patient-specific risk factors identified by logistic regression are: myocardial infarction < 6 months (odds ratio [OR], 4.5; 95% confidence interval [CI], 1.9 to 12.9), emergency surgery (OR, 2.6; 95% CI, 1.2 to 5.6), myocardial infarction >6 months (OR, 2.2; 95% CI, 1.4 to 3.5), heart failure ever (OR, 1.9; 95% CI, 1.2 to 3.0), and rhythm other than sinus (OR, 1.7; 95% CI, 0.9 to 3.2). Inclusion of the planned operative procedure significantly improves the predictive ability of our risk model.
CONCLUSIONS
Five patient-specific risk factors are associated with high risk for cardiac complications in the perioperative period of noncardiac surgery in veterans. Inclusion of the operative procedure significantly improves the predictive ability of the risk model. Overall cardiac complication rates (pretest probabilities) are established for these patients. A simple nomogram is presented for calculation of post-test probabilities by incorporating the operative procedure.
Keywords: cardiac risk, noncardiac surgery, risk index, risk factors, heart disease
A common reason surgeons consult general internists and cardiologists is for preoperative assessment of cardiac risk in patients undergoing noncardiac surgery. Using a multifactorial approach, Goldman et al. prospectively established a multivariate index that was associated with adverse cardiac outcome after surgery.1 Several limitations of this index2 led to its modification by Detsky et al.3 This has been the standard risk model used by clinicians for assessing risk of adverse cardiac events after surgery.
We encountered several difficulties when trying to apply Detsky's index to risk stratify patients at the Dallas Veterans Affairs Medical Center (DVAMC). We did not know if it was appropriate to use the overall complication rates derived from Detsky et al.'s population, as our patients may be different from their patients. Compared with 57% of Detsky et al.'s patients, more than 90% of VAMC patients are men and thus likely to have a higher prevalence of coronary artery disease. Detsky et al. also did not include several surgical procedures (e.g., hernia repair, amputations, rotator cuff repair, etc.) commonly performed at the DVAMC, for which preoperative consultation is frequently requested.
The adverse event rates for specific surgical procedures are largely unknown for the veterans affairs (VA) population, although there are reports by Mangano et al.4 and Ashton et al.5 describing overall complication rates of noncardiac surgery in VAMC patients. In addition, these studies differed considerably from our study. Ashton et al. looked at only one outcome, i.e., myocardial infarction, whereas we wanted to look at all important outcomes. Mangano et al. included a broader range of postoperative events but recorded a very high incidence (46%) of ventricular tachycardia (defined as 5 or more consecutive PVCs at a rate of at least 100 per minute). A majority of these events (97%) were asymptomatic. This incidence of ventricular tachycardia is higher than any other study and it is likely that a large number of these events were diagnosed simply because the patients were undergoing continuous holter monitoring.
We also wanted to test the importance of certain variables in our population that were found to be important in Detsky et al.'s cohort. We wanted to examine if isolated elevation of AST (one of the criteria of poor general medical status) that is common in our patients indicated high cardiac risk. Further, during preoperative assessment it is often easier to determine if the patients had previous congestive heart failure than to establish the history of pulmonary edema. We therefore wanted to examine the importance of congestive heart failure. We also sought to explore the value of additional variables such as diabetes mellitus, hypertension, chronic stable angina, and peripheral vascular disease, which have been found to be important by some investigators but not others.1,4–13 Given all these constraints, conflicting data, and lacking good information in veterans, we wanted to assess the cardiac risk of a broad range of noncardiac procedures and develop a cardiac risk index applicable to VAMC patients.
METHODS
This study was performed at the DVAMC from April 1992 to December 1995. Participants included patients who had known or suspected cardiac disease determined by presence of previous myocardial infarction, angina, coronary revascularization, abnormal coronary angiography, cardiomyopathy, heart failure, arrhythmias, valvular disease, heart murmur, abnormal electrocardiogram (ECG), or chest pain. All patients meeting these criteria were offered study participation. A vast majority of our patients were derived from the General Medicine and Cardiology Preoperative Consult Services. Our patient population is therefore more similar to Detsky's, who included patients with a question of a cardiac problem and different from that of Goldman et al., who studied consecutive patients over the age of 40 years. Because patients' risk differs with time (e.g., patients may acquire new risk variables) and with the operative procedure, patients were eligible to be re-enrolled in the study after a 2-week period free of any cardiac events or after full recovery from an event. The DVAMC institutional review board approved the study. Informed consent was obtained from all participants.
With a conservative projection of a 7.5% complication rate based on literature review, a sample size of 1,134 procedures was estimated to produce 5 end points for each variable included in the model (we had sought to test approximately 17 variables). A total of 1,758 patients were evaluated prior to 1,879 procedures. Of these, 580 never had surgery (no surgery group), 167 were not enrolled (no study group), and 1,011 patients undergoing 1,132 procedures were enrolled. Eleven (1%) patients were excluded after enrollment because of incomplete follow-up. A total of 1,121 operative procedures performed on 1,000 patients constitute the study sample. A single procedure was performed in 894 patients, 2 in 94, 3 in 9, and 4 in 3 patients. Ten procedures were performed on 10 women. The ages of patients ranged from 34 to 88 years (mean 66 ± 8.5 years).
Preoperative Assessment
Patients were evaluated by one of the investigators (RK, WPM, GR, GRH, or HJH) directly or indirectly through supervision of the residents. Evaluations included a detailed medical history, physical examination, and review of old medical records and laboratory tests including creatine kinase (CK) with MB fraction, electrolytes, AST, a chest radiograph, and an ECG with a 1-minute rhythm strip. Data was collected for 19 patients by chart review alone (these patients had been interviewed and assessed but their original preoperative data forms were lost). Patients were specifically evaluated for previous myocardial infarction; Canadian Cardiovascular Society Angina (CCSA) Classes III and IV; stable and unstable angina; alveolar pulmonary edema; congestive heart failure; critical aortic stenosis; nonsinus rhythm or premature atrial contractions on the last preoperative ECG; more than 5 premature ventricular contractions (PVCS) per minute ever; poor general medical status; age; emergent or urgent surgery; diabetes mellitus; hypertension; and peripheral vascular disease. The variables were defined in the same fashion as in Detsky et al.3 with the following exceptions: critical aortic stenosis was defined as calculated valve area of 0.5 cm2/m2by echocardiogram, with a mean gradient of >40 mm Hg and a velocity across the valve of >4 m/sec in a patient with consistent clinical picture. Hypokalemia was defined as a serum level of <3.5 mEq/L. Diabetes was defined as fasting serum glucose level ≥140 mg/dl on more than one occasion requiring treatment with diet or drugs. Hypertension was defined as use of medication to lower blood pressure, or documented systolic or diastolic blood pressure of >160 mm Hg and >90 mm Hg, respectively. Peripheral vascular disease was defined as presence of claudication, rest pain, abnormal angiography, or prior revascularization. Angina was considered stable if it had not increased in any respect, and had not caused decreased activity in the last 3 months. Congestive heart failure was defined as history of dyspnea on exertion; orthopnea; or paroxysmal nocturnal dyspnea with response to diuretics, afterload reduction, or digoxin; or was confirmed by a chest radiograph. Surgery necessary within 12 hours was considered emergent and between 12 to 72 hours urgent.
Postoperative Outcomes Assessment
An ECG was obtained immediately postoperatively. All patients were then assessed daily with a clinical examination, an ECG, and CK with MB fraction from postoperative days 1 through 6, the day of discharge, reoperation, or death (whichever occurred earliest). Postoperative assessment was done by one of the authors blinded to patients' preoperative status. If patients developed cardiac chest pain, heart failure, arrhythmias, hypotension, unexplained altered mental status, elevated CK with CK-MB, or ischemic electorocardiographic changes on routine testing, a series of ECGs and total CKs with MB fraction were obtained every 8 hours for a total of 3 determinations. Chest radiographs were done for clinical suspicion of heart failure or pulmonary edema or at the discretion of the surgeons. These were reviewed by two radiologists, and, in case of disagreement, by a third radiologist. Two physicians blinded to the patient's preoperative status reviewed all postoperative data on patients with suspected adverse cardiac events. A third blinded reviewer or consensus panel of authors (RK, WPM, GR, and GH) resolved controversies.
Outcome Events
Severe perioperative cardiac events were prospectively defined as cardiac death, myocardial infarction, pulmonary edema, cardiac arrest, and nonfatal ventricular tachycardia and ventricular fibrillation. Serious cardiac events included the above, unstable angina, and new or worsened heart failure. Cardiac death was defined as death in the setting of myocardial infarction, ventricular arrhythmias, cardiogenic shock, or if death was sudden and unexplained. Myocardial infarction was diagnosed by the presence of two or more of the following: 1) ECG showing new pathological Q waves in at least 2 leads; loss of R waves; or ST-T waves changes consistent with non–Q-wave infraction; 2) presence of an elevated total creatine kinase, CK-MB, and its ratio to total CK; and 3) cardiac chest pain (all of our patients had elevated total CK, CK-MB and CK-MB to CK ratio). Pulmonary edema and heart failure were diagnosed by radiographic evidence in an appropriate clinical setting. Cardiac arrest was defined as the absence of palpable pulse in an unresponsive patient with no recordable blood pressure with subsequent successful resuscitation. Ventricular tachycardia was defined as consecutive PVCs occurring at a rate of >100 per minute, sustained for >30 seconds and requiring treatment with cardioversion or antiarrhythmics. Ventricular fibrillation was defined by its characteristic electrocardiographic appearance. Unstable angina was defined as ≥30 minutes of chest pain with electrocardiographic changes consistent with ischemia without infarction. Our definitions differed from those of other investigators (see Appendix for details).
Statistical Methods
Using stepwise logistic regression,14 a risk model was developed by examining variables that were significant by univariate analysis using the χ2test or that were deemed clinically important. These included myocardial infarction within 6 months and more than 6 months prior to surgery; CCSA Class III and IV within 2 weeks; unstable angina within 3 months; stable angina; pulmonary edema or congestive heart failure within the past one week or ever; emergency or urgent surgery; nonsinus rhythm; sinus rhythm with premature atrial contractions; >5 premature ventricular contractions per minute ever; poor general medical status; hypertension; and diabetes. Peripheral vascular disease could not be separated from vascular nature of the procedure and was not included. Significance levels of 0.10 were used as thresholds of entry or removal of a variable from the model because a P value of .05 is considered to be too restrictive leading to exclusion of important risk factors.15 The odds ratios and logistic function coefficients associated with each statistically significant factor were determined. For clinical purposes, point values were derived from the regression coefficients.
Model Validation
Several methods are available for model validation, the gold standard being external validation, i.e., testing the model in a different population. However, because of limitation of resources this is difficult to do. The principal methods of internal validation are the cross-validation, split-sample, jack-knife, and bootstrap techniques. With cross-validation the estimates of accuracy vary when the validation process is repeated. The sample sizes are usually too small to use the split-sample technique.16 Further, in a comparison, Gong17 demonstrated that the bootstrap was superior to the cross-validation and the jack-knife techniques. Given all these considerations and the fact that we had only 91 end points to test candidate variables, we chose to validate the model by the bootstrap technique.18
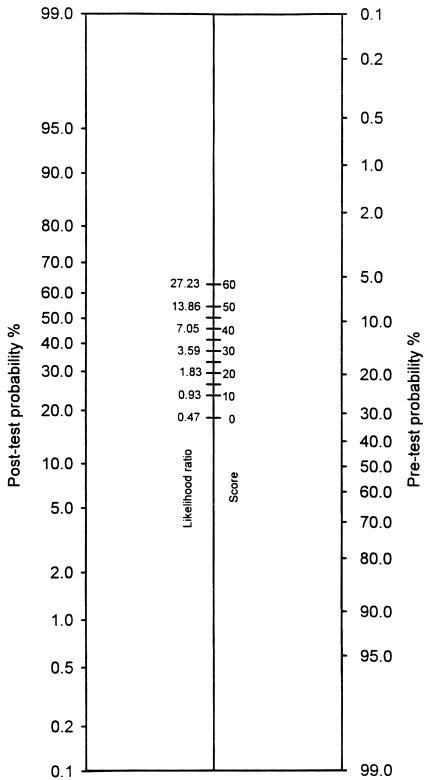
The selected model was compared with those of Goldman et al.,1 Detsky et al.,3 Ashton et al.,5 and Lee et al.13 using area under the curve (AUC) of the receiver operating characteristic (ROC) curve derived by the maximum likelihood method.19 Statistical analysis was performed using SAS, version 6.12 (SAS Institute, Cary, NC). For clinical use we calculated the likelihood ratios for various scores via the “likelihood ratio line” technique.20 A nomogram using specific score values and associated likelihood ratios was constructed to convert pretest probabilities into posttest probabilities.
RESULTS
Baseline Characteristics
The baseline characteristics of the patient population are presented in Table 1).
Table 1.
Patient Characteristics at Baseline
| Characteristic | Patient Procedures*, n | Patient Procedures, % |
|---|---|---|
| Age ≥ 75 years | 159 | 14.2 |
| Myocardial infarction in the past 6 months | 27 | 2.4 |
| Myocardial infarction more than 6 months ago | 469 | 42 |
| Unstable angina in the last 3 months | 37 | 3.3 |
| CCSA Class III in the last 2 weeks | 20 | 1.8 |
| CCSA Class IV in the last 2 weeks | 8 | 0.7 |
| Chronic stable angina | 274 | 24 |
| Acute pulmonary edema in last week | 5 | 0.4 |
| Acute pulmonary edema ever but not last week | 116 | 10.3 |
| Congestive heart failure in last week | 34 | 3.0 |
| Congestive heart failure ever but not in the last week | 247 | 22 |
| Emergency Surgery | 46 | 4.1 |
| Rhythm other than sinus | 112 | 10.0 |
| Normal sinus rhythm with premature atrial contractions | 145 | 12.9 |
| More than 5 premature ventricular contractions per minute ever | 164 | 14.6 |
| Poor general medical status† | 179 | 16 |
| Diabetes Mellitus | 291 | 26 |
| Hypertension | 696 | 62 |
| Suspected critical aortic stenosis | 0 | 0 |
Includes 1121 procedures in 1000 patients.
See text for definition.
Surgical Procedures Studied
We studied 1,121 procedures: 280 vascular (84 aortic, 60 carotid, 68 peripheral, 49 lower limb amputations, and 19 miscellaneous, e.g., AV access); 262 general surgical (146 major intra-abdominal procedures involving the liver, pancreas, small bowel, colon, uterus, gall bladder, and appendix and 116 low-risk procedures, e.g., repair uncomplicated hernias, mastectomy, gastrostomy, thyroidectomy, etc); 216 orthopedic (136 major procedures involving replacement or arthroplasty of the hip, knee, shoulder, elbow, and ankle and procedures involving the spine; and 80 low-risk procedures such as arthroscopic surgery, rotator cuff repair, and operations involving the hand, wrist, and long bones); 162 urologic (44 high-risk intra-abdominal procedures and 118 low-risk transuretheral, percutaneous, or perineal procedures); 42 thoracic (31 thoracotomy and 11 nonthoracotomy procedures); 69 otorhinolaryngologic; 39 neurologic; 30 ophthalmologic, 13 plastic; and 8 maxillofacial procedures.
Outcome Events
A total of 91 procedures resulted in serious cardiac complications. Thirty-one patients had myocardial infarction (with 7 cardiac deaths). Thirty-nine procedures were complicated by pulmonary edema with 1 additional cardiac death (5 patients had myocardial infarction as well). A total of 39 procedures were complicated by symptomatic heart failure, 23 having heart failure as the only complication. Five patients had ventricular tachycardia. All 5 patients had other severe events as well (1 patient had a myocardial infarction and pulmonary edema, 2 suffered myocardial infarction, and 2 developed pulmonary edema). Two patients had cardiac arrest (1 had pulmonary edema as well) and 2 patients had unstable angina. Table 2 shows the distribution of procedures and overall complication rates for different surgical procedures. By using a χ2statistic and applying the Bonferroni correction for multiple comparisons, the severe and serious complication rates are significantly different (P < .0083) for the following comparisons: 1) very-high- versus high-, intermediate-, and low-risk procedures; and 2) high-risk versus intermediate- and low-risk procedures. The severe complication rate for intermediate-risk surgery is also significantly higher than for low-risk surgery but there is no significant difference in the serious complication rates for the two categories.
Table 2.
Prior Probabilities for Various Types of Surgery
| Surgical Risk Category | Severe Cardiac* Complications (%) | Serious Cardiac† Complications (%) |
|---|---|---|
| Very-high-risk surgery | ||
| Aortic | 20/84 (23.8) | 27/84 (32.0) |
| High-risk surgery | 38/417 (9.1) | 52/417 (12.5) |
| Vascular | 18/196 (9.2) | 23/196 (11.7) |
| Carotid | 5/60 (8.3) | 6/60 (10.0) |
| Peripheral | 8/68 (11.8) | 10/68 (14.7) |
| Miscellaneous‡ | 5/68 (7.4) | 7/68 (10.3) |
| Nonvascular§ | ||
| Intra-abdominal/intrathoracic | 20/221 (9.0) | 29/221 (13.1) |
| Intermediate-risk surgery∥ | 7/244 (2.9) | 8/244 (3.3) |
| Low-risk surgery¶ | 1/376 (0.27) | 4/376 (1.1) |
Cardiac death, myocardial infarction, alveolar pulmonary edema, cardiac arrest, and nonfatal ventricular tachycardia and fibrillation.
Above plus unstable angina and new or worsened congestive heart failure without alveolar pulmonary edema.
All lower extremity amputations, AV access procedures, Hickman catheter placement, etc.
Major intra-abdominal general surgery and urologic procedures and thoracic procedures requiring thoracotomy (see text for details).
Neurosurgical, ENT, and major orthopedic procedures (see text for details).
Ophthalmologic, maxillofacial, and plastic and low-risk orthopedic, urologic, general surgery and nonthoracotomy thoracic procedures (see text for details).
The severe and serious complication rates for aortic surgery are significantly higher than for high-risk, intermediate-risk and low-risk surgery (P < .0083 with the Bonferroni correction).
The severe and serious complication rates for high-risk surgery are significantly higher than for intermediate- and low-risk surgery (P < .0083 with the Bonferroni correction).
The severe complication rate for intermediate-risk surgery is significantly higher than for low-risk surgery (P < .0083 with the Bonferroni correction). There is no significant difference between the serious complication rates for intermediate- and low-risk surgery.
The univariate correlation of different variables with adverse cardiac outcome is presented in Table 3. Age was examined both as a continuous variable and as a dichotomous variable with a cut point at the age of 75 years. As a continuous variable, age was found to be insignificant. When examined as a dichotomous variable, it appeared that patients ≥75 years were at lower risk. However, fewer older patients had higher-risk procedures. Of patients <75 years, 46% had high- or very–high-risk surgery compared with 34% of the older patients (P = .003). In addition, the complication rates for surgical procedures in various risk categories were not significantly different for patients above or below age 75 (Cochran-Mantel-Haenszel test, P = .377).21 Therefore, age was not included in the logistic regression. Variables identified by logistic regression to have a statistically significant and independent correlation with serious cardiac complications and their point values are shown in Table 4 Variables correlating with severe events were identical. Figure 1 displays the nomogram for obtaining post-test probabilities given specific pretest probabilities for the new index.
Table 3.
Univariate Correlates of Adverse Cardiac Outcome
| Adverse Cardiac Outcome | ||||
|---|---|---|---|---|
| Preoperative Risk Variable | Variable Present (%) | Variable Absent (%) | P Value | OR (95% CI) |
| MI < 6 mo | 8/27 (30) | 83/1094 (8) | .001 | 5.1 (2.2 to 12.1) |
| MI ever but not within 6 mo | 52/469 (11) | 39/652 (6) | .002 | 2.0 (1.3 to 3.0) |
| CCSA Class III within 2 wk | 3/20 (15) | 88/1101 (8) | .255 | 2.0 (0.6 to 7.1) |
| CCSA Class IV within 2 wk | 3/8 (38) | 88/1113 (8) | .002 | 7.0 (1.6 to 29.7) |
| Unstable angina < 3 mo | 4/37 (11) | 87/1084 (8) | .542 | 1.4 (0.5 to 4.0) |
| Chronic stable angina | 25/274 (9) | 66/847 (8) | .483 | 1.2 (0.7 to 1.9) |
| APE within 1 wk | 1/5 (20) | 90/1116 (8) | .330 | 2.9 (0.3 to 25.8) |
| APE ever but not within 1 wk | 20/116 (17) | 71/1005 (7) | .001 | 2.7 (1.6 to 4.7) |
| CHF within 1 wk | 8/34 (24) | 83/1087 (8) | .001 | 3.7 (1.6 to 8.5) |
| CHF ever but not within 1 week | 33/247 (13) | 58/874 (7) | .001 | 2.2 (1.4 to 3.4) |
| Emergency surgery | 11/46 (24) | 80/1075 (7) | .001 | 3.9 (1.9 to 8.0) |
| Rhythm other than sinus on last ECG | 16/112 (14) | 75/1009 (7) | .012 | 2.1 (1.2 to 3.7) |
| NSR with PACs | 9/145 (6) | 82/976 (8) | .367 | 0.7 (0.4 to 1.5) |
| More than 5 PVCs ever | 13/164 (8) | 78/957 (8) | .923 | 1.0 (0.5 to 1.8) |
| Poor general medical status | 21/179 (12) | 70/942 (7) | .053 | 1.7 (1.0 to 2.8) |
| Diabetes mellitus | 26/291 (9) | 65/830 (8) | .553 | 1.2 (0.7 to 1.9) |
| Hypertension | 56/696 (8) | 35/425 (8) | .910 | 1.0 (0.6 to 1.5) |
| Age ≥ 75 y | 7/159 (4) | 84/962 (9) | .064 | 0.5 (0.2 to 1.1) |
MI, myocardial infarction; CCSA, Canadian Cardiovascular Society angina; APE, acute pulmonary edema; CHF, congestive heart failure; PAC, premature atrial contraction; NSR, normal sinus rhythm; PVC, premature ventricular contraction; OR, odds ratio.
Table 4.
Computation of the Cardiac Risk Index
| Preoperative Risk Variable | Points | Logistic Function Coefficient | Odds Ratio (95% CI) |
|---|---|---|---|
| −5.1916 | |||
| MI within 6 months | 25 | 1.5849 | 4.9 (1.9 to 12.9) |
| Emergency surgery | 15 | 0.9550 | 2.6 (1.2 to 5.6) |
| MI ever, but not within 6 months | 10 | 0.7849 | 2.2 (1.4 to 3.5) |
| Congestive heart failure ever, but not within 1 week | 10 | 0.6272 | 1.9 (1.2 to 3.0) |
| Rhythm other than sinus on last ECG | 10 | 0.5399 | 1.7 (0.9 to 3.2) |
The minimum score for a patient is zero and the maximum is 60.
A patient with history of a remote myocardial infarction and another one within 6 months scores 25 points, not 35.
Significance levels of 0.10 were used as thresholds of entry or removal of a variable from the model.
FIGURE 1.
Likelihood ratio nomogram. Select a point reflecting the overall complication rate on the pretest side of the nomogram. Draw a line connecting this point to a point on the center column that reflects the patient's index score and associated likelihood ratio. Extend this line to intersect the post-test side of the nomogram. The point of intersection gives the post-test probability, i.e., the risk of perioperative cardiac complications.
Model Validation
Using the bootstrap technique with 100 replications of resampling our data, the frequency of selection of variables was as follows: emergency surgery (95%), infarction (93%), congestive heart failure (78%), nonsinus rhythm (60%), pulmonary edema (21%), and the remainder of the variables (≤5%). The median AUC was 0.67, which compares well with the observed value of 0.70.
Comparison with Other Indices
The performance of our model was compared with several other models with use of ROC analysis by the maximum likelihood method. The AUCs for the VA index for serious and severe complications without taking the operative procedure into consideration are 0.70 ± 0.03 and 0.71 ± 0.04, respectively (Table 5). The AUC for the VA model for serious complications is significantly different from the Goldman model, but for severe complications it is not significantly different from any of the other models. To make a more stringent comparison we also compared the bootstrapped AUC of the VA model for serious complications with the other models. It was not feasible to compare the bootstrapped AUC of one model with the nonbootstrapped AUC of another model; therefore, the bootstrapped AUCs for all the other models were calculated. Because no variable selection is occurring for these models, their bootstrapped and nonbootstrapped AUCs are very similar and minimally handicapped. As seen in Table 5, the bootstrapped AUC for the VA model is significantly different from the Goldman and the Detsky models but not from the Ashton and the Lee models. Further, when the types of the operative procedures by using their regression coefficients are incorporated into the VA model, the AUCs for serious and severe complications change to 0.83 ± 0.02 and 0.84 ± 0.03, respectively. These are significantly different from the AUCs without incorporation of the surgical procedure (P value < .0001 for both) and are also significantly different from all the other models (Table 6). However, if we incorporate the operative procedure into the Detsky model (the only other model that does not include any surgical procedures), there is no significant difference between the VA and the Detsky models.
Table 5.
Comparison of VA with Other Models (without Consideration of the Operative Procedure)
| Serious Complications | Severe Complications | Serious Complications Bootstrapped AUCs | ||||
|---|---|---|---|---|---|---|
| Model | AUC | P Value | AUC | P Value | AUC | P Value |
| DVAMC | 0.70 ± 0.03 | — | 0.71 ± 0.04 | — | 0.67 ± 0.03 | — |
| Goldman et al.1 | 0.62 ± 0.03 | 0.033 | 0.66 ± 0.04 | 0.19 | 0.62 ± 0.03 | 0.025 |
| Detsky et al.3 | 0.64 ± 0.03 | 0.09 | 0.66 ± 0.04 | 0.17 | 0.63 ± 0.03 | 0.012 |
| Ashton et al.5 | 0.65 ± 0.03 | 0.10 | 0.67 ± 0.02 | 0.17 | 0.64 ± 0.03 | 0.096 |
| Lee et al.13 | 0.74 ± 0.03 | 0.22 | 0.73 ± 0.03 | 0.33 | 0.73 ± 0.03 | 0.053 |
Severe complications include cardiac death, myocardial infarction, alveolar pulmonary edema, cardiac arrest, nonfatal ventricular tachycardia, and fibrillation.
Serious complications include above plus unstable angina and new or worsened congestive heart failure without pulmonary edema.
AUC, area under the curve.
The AUC for the DVAMC model is the reference value with which all others are being compared.
Table 6.
Comparison of VA with Other Models (With Consideration of the Operative Procedure)
| Serious Complications | Severe Complications | |||
|---|---|---|---|---|
| Model | AUC | P Value | AUC | P Value |
| VA | 0.83 ± 0.02 | — | 0.84 ± 0.03 | — |
| Goldman et al.1 | 0.62 ± 0.03 | 0.0001 | 0.66 ± 0.04 | 0.001 |
| Detsky et al.3* | 0.64 ± 0.03 | 0.0001 | 0.66 ± 0.04 | 0.001 |
| Detsky et al.3† | 0.82 ± 0.02 | 0.085 | 0.83 ± 0.03 | 0.305 |
| Ashton et al.5 | 0.65 ± 0.03 | 0.0001 | 0.67 ± 0.02 | 0.001 |
| Lee et al.13 | 0.74 ± 0.03 | 0.01 | 0.73 ± 0.03 | 0.014 |
Detsky index without incorporation of the operative procedure.
Detsky index with incorporation of the operative procedure. (All the other indices incorporate certain surgical procedures in their models)
Severe complications include cardiac death, myocardial infarction, alveolar pulmonary edema, cardiac arrest, nonfatal ventricular tachycardia and fibrillation
Serious complications include above plus unstable angina and new or worsened congestive heart failure without pulmonary edema.
AUC, area under the curve
The AUC for the DVAMC model is the reference value with which all others are being compared.
The median (first quartile, third quartile) DVAMC risk index scores were 10 (0,10) for the study, 10 (0,10) for the no surgery, 0 (0,10) for the excluded, and 0 (0,10) for the no study groups respectively. The difference was statistically significant between the no study and the study groups (Wilcoxon rank sum test P = .001, adjusted for multiple comparisons by the Tukey-Kramer method).22
DISCUSSION
Using logistic regression analysis in a study of 1,121 noncardiac surgical procedures at a large urban VAMC, we have identified 5 patient-specific variables that are associated with serious and severe perioperative cardiac complications: myocardial infarction within 6 months, a remote infarction, emergency surgery, history of congestive heart failure, and nonsinus rhythm. Despite inclusion of different types of patients, operative procedures, surveillance strategies, outcomes, preoperative variables, definitions of outcomes, and likely definitions of preoperative variables, similar risk factors are found to be important by several investigators.1,3,5,9,12,13
Effect of the Operative Procedure
In addition, the type of the operative procedure was found to have a profound effect on adverse cardiac event rate in our study. As shown, the incorporation of the operative procedure led to a significant improvement in the AUCs by the ROC analysis from 0.70 to 0.83 for serious and from 0.71 to 0.84 for severe cardiac complications. Further, with incorporation of the operative procedure, the AUC of the Detsky index as applied to our patients also improved substantially from 0.66 to 0.83 for severe complications and from 0.64 to 0.82 for serious complications. These operative procedure effects highlight a unique contribution of our index and demonstrate the critical importance of including specific operative intervention in prediction models.
The differential risk associated with various surgical procedures has been recognized for some time. In 1972, Tarhan et al.23 reported that the reinfarction rate following operations on the thorax and upper abdomen was 3 times that following operations on other sites. A very low rate of myocardial infarction has been reported after ophthalmic surgery in patients with (0.3%) and without (0.01%) coronary artery disease.24 In 195 patients with prior infarction who underwent 288 ophthalmic procedures, no postoperative reinfarctions were reported.24 This contrasts with a 6.1% postoperative reinfarction rate in other general surgical patients from the same institution.8 Detsky et al. reported a postoperative infarction rate of 4.8% in patients undergoing major procedures and 0.5% in patients undergoing minor procedures. The severe and serious complication rates of 10% and 16% with major surgery were substantially higher than the 1.6% severe and 2.1% serious complication rates with minor surgery.3 More recently, a very low rate of myocardial infarction (0.03%) was reported in 38,598 patients undergoing 45,090 consecutive ambulatory procedures.25 Mortality after vascular surgery is particularly high26 and 40% to 60% of the patients die from cardiac causes.7,27
Over time, various investigators have incorporated restricted categories of operative procedures in cardiac risk assessment models with limited success. Goldman et al. assigned a distinct point value for intraperitoneal, intrathoracic, and aortic operations. However, Jeffrey et al.2 reported substantially higher cardiac event rates in all score classes in a cohort of 99 consecutive patients undergoing abdominal aortic surgery than in Goldman et al.'s original cohort. Thus the point value assigned for aortic surgery may have failed to fully reflect the risk associated with it. Ashton et al.5 included a planned vascular procedure in their model, whereas Lee et al.13 incorporated high-risk surgery defined as intraperitoneal, intrathoracic, or suprainguinal vascular procedures. The ACC/AHA guidelines for perioperative cardiovascular evaluation for noncardiac surgery advise clinicians to take surgery-specific risk factors into consideration and include aortic, other major, and peripheral vascular procedures in the high-risk category.28 However the risk of different vascular, intraperitoneal, and intrathoracic procedures may not be the same. In our study, the severe complication rate of 24% associated with aortic surgery is significantly higher than the 9% severe complication rate associated with intra-abdominal, intrathoracic, and other vascular procedures. It is likely that with incorporation of more categories of operative procedures, the predictive ability of various models may improve substantially. We elected to use a Bayesian approach that allows incorporation of risk associated with any number of specific operative procedures for which the pretest probability is known.
Several risk factors considered important by others1,3 were found to be unimportant in our study: age, CCSA Classes III and IV, unstable angina, >5 PVCs at any time prior to surgery, preoperative congestive heart failure, poor general medical status including an elevated AST and bedridden status from noncardiac causes, stable angina, diabetes, and hypertension. However, our older patients underwent less stressful procedures. Also, only the healthiest veterans may survive to age ≥75 years. Other investigators have also found age to be unimportant.6,8,9,13 Most of our patients with CCSA Classes III and IV and unstable angina underwent various interventions including preoperative medication adjustment, coronary revascularization, and/or aggressive perioperative monitoring. Increased risk associated with frequent PVCs described in the early literature may have been due to aggressive antiarrhythmic use in that era. In our study, all patients had their general medical, cardiac, renal, and pulmonary statuses optimized preoperatively. Therefore, poor general medical status may have been found to pose no increased risk. Patients with decompensated CHF may have been more rigorously managed in the perioperative period. Bedridden patients with elective high-risk procedures were adequately screened for coronary artery disease. Patients with diabetes may have other associated risk factors, e.g., coronary artery disease, that overshadow its importance. Importance of hypertension in studies done in the 1960s and 1970s6,8 but not in later studies1,5,9,12 may be due to suboptimal treatment in the earlier period. Finally we had no patients with critical aortic stenosis to assess its importance.
Our study has several strengths. Postoperatively, all patients were followed with a daily examination, ECG, CK, and CK-MB. It is therefore quite unlikely that any significant outcomes were missed. We used total CK, CK-MB, and their ratio for diagnosis of myocardial infarction. This approach is extremely sensitive29 and 99% specific.30 The assessors of postoperative course were blinded to preoperative assessment, thus minimizing expectation bias. We had 91 end points to examine 18 variables, giving us at least 5 end points per variable as recommended by Lachenbruch.31 Goldman et al., Larsen et al., Shah et al., and Ashton et al. had only 0.8, 1.0, 1.7, and 1.3 end points respectively per variable examined. The interobserver variability was minimized as chest radiographs confirmed heart failure and pulmonary edema. The model was validated by bootstrapping which allows the use of the entire data for model selection unlike methods that split the data and therefore have fewer end points to derive the model.32 The VAMCs have an ethnically diverse population and there is no differential care based on insurance status. The results are likely generalizable to the VA system which provides care to more than 3 million veterans.
Our study also has several limitations. All eligible patients were not enrolled, and the nonenrolled appear to be at lower risk. The importance of CCSA Class III and IV, unstable angina, critical aortic stenosis, and decompensated congestive heart failure cannot be completely excluded as there were few patients with these variables and most were stabilized preoperatively. Our model does not include the effects of β -blockers as the study was performed before their benefits were recognized.33 The VAMC population has fewer women and a higher prevalence of smoking, drinking, and comorbid conditions and a lower socioeconomic status limiting the generalizability of the study to other populations. Further the model was validated by the bootstrap technique while the most stringent method to test the accuracy of the model is that of external validation, i.e., application of the model to a different population. Also, it needs to be emphasized that our model was retrospectively derived and needs prospective validation in the veteran and other populations.
Because the comparison of the VA cardiac risk index to other indices was conducted within the same data set that was used to generate the VA index, the latter would be expected to perform better in this data set and thus its success may be overestimated. The predictive accuracy of the index is likely to decrease as it is tested in different populations as recently demonstrated by Gilbert et al.34 The erosion of accuracy occurs as a result of several factors: the patient populations are different and have different risk factors; operative procedures included are distinct; some authors include major procedures only while others include minor procedures as well; and outcomes considered are disparate, e.g., some1,3 include cardiac death as an outcome whereas others34 include all deaths. Unstable angina was included both by Detsky et al. and Gilbert et al. but not by Goldman et al. Definitions of variables differ as well. Detsky et al. defined unstable angina as 30 minutes of chest pain with persistent ECG changes whereas Gilbert et al. defines it as “typical symptoms with new ECG changes compatible with ischemia” with no particular requirement for duration of chest pain. There are likely subtle differences in the definitions of other events as well. The surveillance strategies differ also, some studies including events by chart review only while others using more stringent follow-up. Lastly the predictive accuracy can erode in the same cohort of patients as recently seen in the study of Lee et al.13 Of the 6 risk factors found to be important in the derivation set, only 4 were found to correlate in the validation set.
CONCLUSIONS
We have identified 5 patient-specific clinical variables that are independently associated with adverse cardiac outcomes in veterans after noncardiac surgery: myocardial infarction within 6 months, a remote infarction, emergency surgery, history of congestive heart failure, and nonsinus rhythm. In addition, the type of the operative procedure also has a profound effect on the cardiac risk. To incorporate the risk associated with the surgical procedures, we have constructed a nomogram (Figure 1), which allows estimation of an individual patient's risk for a specific surgical procedure. Critical aortic stenosis, CCSA Class III or IV, unstable angina, and decompensated heart failure were insignificant risk variables. However, we had few patients with these variables and most had treatment optimized prior to surgery. Age was also an unimportant risk factor. Patients with more than 35 points on the VA cardiac risk index were at especially high risk. While we expect this index to be generalizable to other VAMCs and institutions with similar populations, confirmatory studies are needed.
Acknowledgments
The authors thank Dr. Mark Feldman for his advice, encouragement, and critical review of the grant application and the manuscript; Drs. Ward M. Terry, H.M. Yuan, and Luta B. Roberts for reviewing chest radiographs; Dr. Patricia Bergen for help in classifying various surgical procedures into concise categories; Carol Albertson, RN, for help in data collection; Janet P. Smith for data management; Lynne E. Kendrick for data entry; Sandra M. Hawes and Teresa D. Autrey for preparation of the manuscript; the many surgeons, ward secretaries, and nurses in the recovery and intensive care units; and ECG technicians and the Laboratory and Research Services at the Dallas VA North Texas Health Care System for their invaluable help with this project.
Investigator Initiated Merit Review Proposal #91–017 was funded by Health Services Research and Development Service, Department of Veterans Affairs.
APPENDIX
Definitions of Outcome
| Goldman | Detsky | Larsen | Shah | Ashton | Lee | Gilbert | Current Study |
|---|---|---|---|---|---|---|---|
| Myocardial infarction | |||||||
| Transmural MI | |||||||
| New Q waves at least 0.04 seconds in duration and 1mm or more in depth. | New unequivocal Q waves at least 0.04 seconds in duration or loss of R waves in the precordial leads OR persistent ST-T wave abnormalities consistent with sub endocardial infarction in the presence of a positive CPK-MB determination. | Autopsy findings OR 2 of the following 3 criteria: (a) typical chest pain; (b) CK-MB rise above twice the upper reference limit; (c) Characteristic ECG changes i.e. Q waves with a duration of 0.04 or new ST-T abnormalities persisting 6 days with no explanation other than ischemia. | Increase in serum levels of CK-MB isoenzymes with the presence of chest pain or ECG evidence of sub endocardial or transmural myocardial infarction. | New Q-QS patterns and serial changes in CK-MB levels. Patients with equivocal ECGs or enzymes underwent technetium pyrophosphate infarct scintigraphy. | Peak CKMB >5% of an elevated total CK OR Peak CK-MB was >3% of an elevated total CK in the presence of ECG changes consistent with ischemia or infarction (Above definition applies when CK-MB estimated by ion-exchange chromatography assay). When CK MB mass assay was used the following definition was used: Increased CK MB above normal range with a ratio of CK-MB total CK >0.0278 or >0.0167 in the presence of ECG changes. | Acute ECG changes and an elevation of CK-MB to at least 5.0 U/L with a MB index at least 0.4. | Presence of two of the following: (1) ECG showing new pathological Q waves in at least two leads; loss of R waves; or ST-T wave changes consistent with non–Q wave infarction; (2) presence of an elevated total CK, CK-MB and CK-MB to CK ratio; (3) cardiac chest pain. |
| Nontransmural MI | |||||||
| New ST segment depression or T wave inversion persistent for 72 hours associated either with cardiac chest pain or with elevated AST, CPK or when available CPK isoenzymes that could not be explained on the basis of the surgical course itself. | |||||||
| OR | |||||||
| new ST segment depression of 1mm or more and symmetrical T wave inversion persistent for at least 7 days | |||||||
| Pulmonary edema | |||||||
| Classic chest xray changes or respiratory distress and rales at least three fourths of the way up the lung fields that improved promptly with diuretic therapy. | Same as Goldman. | Definite clinical signs with prompt diuretic response or by typical xray findings. | Not included. | Not included. | Chest radiograph in a plausible clinical setting. | Compatible clinical OR Radiographic findings. | Compatible clinical and radiological findings. |
| Cardiac death | |||||||
| Death from an arrhythmia or refractory low cardiac output that was not part of an inexroable downhill course caused by a non-cardiac cause. | Same as Goldman. | Careful review of all details of the case at an interdisciplinary conference. | Death after MI, documented cardiac dysrrythmias or cardiogenic shock or when death was sudden and unexplained | Not included. | No specific definition. | Not included (Included all-cause mortality). | Death in the setting of MI, ventricular arrythmias, cardiogenic shock, or if the death was sudden and unexplained. |
| Ventricular tachycardia and fibrillation | |||||||
| Electrocardiograms available for review. | Ventricular tachycardia or fibrillation necessitating counter shock with a nonfatal outcome. | ECG confirmed for more than 8 seconds. | Not included. | Not included. | No specific definition. | Not included. | Ventricular tachycardia defined as consecutive PVCs occurring at a rate of >100 per minute, lasting for >30 seconds and requiring treatment with cardioversion or antiarrhythmics. Ventricular fibrillation was defined by the characteristic ECG appearance. |
| New or worsened CHF | |||||||
| Not included. | Presence of new respiratory distress, S3, jugular venous distension and a new chest xray finding of pulmonary vascular redistribution or interstitial pulmonary edema. | Not included. | Not included. | Not included. | Not included. | Not included. | Compatible clinical and radiological findings. |
| Unstable angina/coronary insufficiency | |||||||
| Not included. | More than 30 minutes of chest pain with persistent ECG abnormalities consistent with sub- endocardial ischemia in the absence of a positive CK-MB determination. | Not included. | Not included. | Not included. | Not included. | Typical symptoms in a patient with ECG changes compatible with ischemia. | More than 30 minutes of chest pain with ECG changes consistent with ischemia without infarction. |
| Primary cardiac arrest | |||||||
| Not included. | Not included. | Not included. | Not included. | Not included. | No specific definition. | Not included. | Absence of palpable pulse and recordable blood pressure in an unresponsive patient with subsequent resuscitation. |
| Complete heart block | |||||||
| Not included. | Not included. | Not included. | Not included. | Not included. | No specific definition. | Not included. | Not included. |
REFERENCES
- 1.Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–50. doi: 10.1056/NEJM197710202971601. [DOI] [PubMed] [Google Scholar]
- 2.Jeffrey CC, Kunsman J, Cullen DJ, Brewster DC. A prospective evaluation of cardiac risk index. Anesthesiology. 1983;58:462–4. doi: 10.1097/00000542-198305000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Detsky AS, Abrams HB, McLaughlin JR, et al. Predicting cardiac complication in patients undergoing non-cardiac surgery. J Gen Intern Med. 1986;1:211–9. doi: 10.1007/BF02596184. [DOI] [PubMed] [Google Scholar]
- 4.Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo MS. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. N Engl J Med. 1990;323:1781–8. doi: 10.1056/NEJM199012273232601. [DOI] [PubMed] [Google Scholar]
- 5.Ashton CM, Peterson NJ, Wray NP, et al. The incidence of perioperative myocardial infarction in men undergoing noncardiac surgery. Ann Intern Med. 1993;118:504–10. doi: 10.7326/0003-4819-118-7-199304010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Skinner JF, Pearce ML. Surgical risk in the cardiac patient. J Chronic Dis. 1964;17:57–72. doi: 10.1016/0021-9681(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 7.Cooperman M, Pflug B, Martin EW, Jr, Evans WE. Cardiovascular risk factors in patients with peripheral vascular disease. Surgery. 1978;84:505–9. [PubMed] [Google Scholar]
- 8.Steen PA, Tinker JH, Tarhan S. Myocardial reinfarction after anesthesia and surgery. JAMA. 1978;239:2566–70. doi: 10.1001/jama.239.24.2566. [DOI] [PubMed] [Google Scholar]
- 9.Larsen SF, Olesen KH, Jacobsen E, et al. Prediction of cardiac risk in non-cardiac surgery. Eur Heart J. 1987;8:179–85. doi: 10.1093/oxfordjournals.eurheartj.a062246. [DOI] [PubMed] [Google Scholar]
- 10.Foster ED, Davis KB, Carpenter JA, Abele S, Fray D. Risk of noncardiac operation in patients with defined coronary disease: the Coronary Artery Surgery Study (CASS) registry experience. Ann Thorac Surg. 1986;4:42–50. doi: 10.1016/s0003-4975(10)64494-3. [DOI] [PubMed] [Google Scholar]
- 11.Eagle KA, Coley CM, Newell JB, et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110:859–66. doi: 10.7326/0003-4819-110-11-859. [DOI] [PubMed] [Google Scholar]
- 12.Shah KB, Kleinman BS, Rao TLK, Jacobs HK, Mestan K, Schaafsma M. Angina and other risk factors in patients with cardiac diseases undergoing noncardiac operations. Anesth Analg. 1990;70:240–7. doi: 10.1213/00000539-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 14.Hosmer D, Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley and Sons; 1989. [Google Scholar]
- 15.Steyerberg EW, Eijkemans MJ, Harrell FE, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–79. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the cox regression model. Stat Med. 1992;11:2093–109. doi: 10.1002/sim.4780111607. [DOI] [PubMed] [Google Scholar]
- 17.Gong G. Cross-validation, the jackknife and the bootstrap: excess error estimation in forward logistic regression. J Am Stat Assoc. 1986;81:108–13. [Google Scholar]
- 18.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- 19.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 20.Interpretation of diagnostic data: 5. How to do it with simple maths. Can Med Assoc J. 1983. pp. 947–54. No authors listed. [PMC free article] [PubMed]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 22.Kramer CY. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1956;12:307–10. [Google Scholar]
- 23.Tarhan S, Moffit EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220:1451–4. [PubMed] [Google Scholar]
- 24.Backer CL, Tinker JH, Robertson DM, Vlietstra RE. Myocardial reinfarction following local anesthesia for ophthalmic surgery. Anesth Analg. 1980;59:257–62. [PubMed] [Google Scholar]
- 25.Warner MA, Shields SE, Chute CG. Major morbidity and mortality within 1 month of ambulatory surgery and anesthesia. JAMA. 1993;270:1437–41. doi: 10.1001/jama.270.12.1437. [DOI] [PubMed] [Google Scholar]
- 26.Fleisher LA, Eagle KA, Shaffer T, Anderson GF. Perioperative and long term mortality rates after major vascular surgery: the relationship to preoperative testing in Medicare population. Anesth Analg. 1999;89:849–55. doi: 10.1097/00000539-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Hertzer NR. Fatal myocardial infarction following abdominal aortic aneurysm resection: three hundred and forty-three patients followed 6–11 years postoperatively. Ann Surg. 1980;192:667–73. doi: 10.1097/00000658-198019250-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eagle KA, Brundage BH, Chaitman BR, et al. Guidelines for perioperative evaluation for noncardiac surgery. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol. 1996;27:910–48. doi: 10.1016/0735-1097(95)99999-x. [DOI] [PubMed] [Google Scholar]
- 29.Mair J, Morandell D, Genser N, Lechleitner P, Dienstl F, Puschendorf B. Equivalent early sensitivities of myoglobin, creatine kinase MB mass, creatine kinase isoform ratios, and cardiac troponins I and T for acute myocardial infarction. Clin Chem. 1995;41:1266–72. [PubMed] [Google Scholar]
- 30.Adams JE, Sicard GA, Allen BT, et al. Diagnosis of perioperative myocardial infarction with measurement of cardiac troponin I. N Engl J Med. 1994;330:670–4. doi: 10.1056/NEJM199403103301003. [DOI] [PubMed] [Google Scholar]
- 31.Lachenbruch P. Some misuses of discriminant analysis. Methods Inf Med. 1977;16:255–8. [PubMed] [Google Scholar]
- 32.Harrel F, Lee K, Mark D. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Poldermans D, Boersma E, Bax JJ, et al. The effect of Bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341:1789–94. doi: 10.1056/NEJM199912093412402. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert K, Larocque BJ, Patrick LT. Prospective evaluation of cardiac risk indices for patients undergoing noncardiac surgery. Ann Intern Med. 2000;133:356–9. doi: 10.7326/0003-4819-133-5-200009050-00011. [DOI] [PubMed] [Google Scholar]