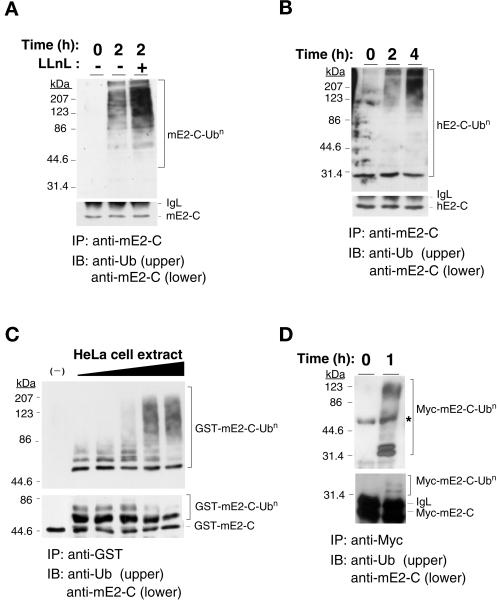
Figure 4.
Cell cycle-dependent polyubiquitination of mammalian E2-C. (A) Mouse NIH 3T3 cells were arrested at metaphase by nocodazole treatment and then were incubated in nocodazole-free medium for the indicated times in the absence or presence of the proteasome inhibitor LLnL at a concentration of 250 μM. Cell lysates were prepared and subjected to immunoprecipitation (IP) with anti-mE2-C, and the resulting precipitates were subjected to immunoblot analysis (IB) with either antiubiquitin (upper panel) or anti-mE2-C (lower panel). The positions of mE2-C, polyubiquitinated mE2-C (mE2-C-Ubn), and immunoglobulin light chain (IgL) are indicated. (B) Human HeLa cells were subjected to metaphase arrest, were incubated for the indicated times in nocodazole-free medium in the presence of LLnL, and were subjected to immunoprecipitation and immunoblot analysis, as in (A). The positions of hE2-C, monoubiquitinated hE2-C (hE2-C-Ub1), polyubiquitinated hE2-C (hE2-C-Ubn), and IgL are indicated. (C) A recombinant GST fusion protein of mE2-C was incubated for 1 h at 37°C in the absence (–) or presence of various concentrations (0.25 μg/μl, 0.5 μg/μl, 1.0 μg/μl, 2.5 μg/μl, and 5.0 μg/μl) of cell extract prepared from HeLa cells 2 h after release from nocodazole-induced arrest. The fusion protein then was immunoprecipitated with anti-GST and ws subjected to immunoblot analysis with antiubiquitin (upper panel) or anti-mE2-C (lower panel). (D) Cell lysates were prepared from NIH 3T3 cells expressing Myc-tagged mE2-C that had been arrested at metaphase by nocodazole treatment and incubated for 0 or 1 h in nocodazole-free medium. The lysates were subjected to immunoprecipitation with anti-Myc, and the resulting precipitates were boiled in SDS-containing buffer and were subjected to reimmunoprecipitation with anti-Myc. The final precipitates then were subjected to immunoblot analysis with antiubiquitin (upper panel) or anti-mE2-C (lower panel). The asterisk indicates a nonspecific band.