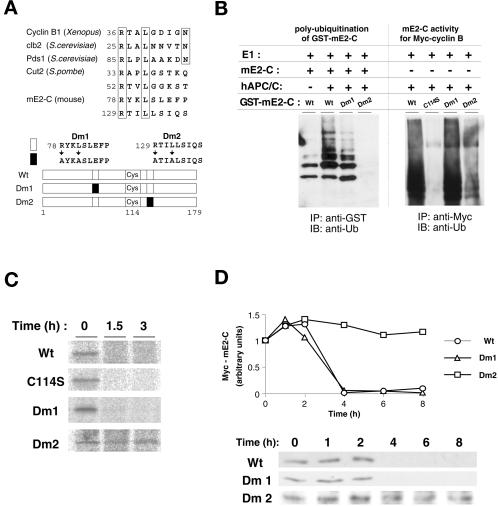
Figure 6.
Destruction boxes are involved in the ubiquitination and stability of mammalian E2-C. (A) The amino acid sequences of the destruction boxes of cyclin B1 (Xenopus laevis), Clb2 (Saccharomyces cerevisiae), Pds1 (S. cerevisiae), Cut2 (Schizosaccharomyces pombe), and mE2-C (Mus musculus) are aligned in the upper panel. Amino acids conserved among these proteins are boxed. Residue numbers are shown at the beginning of each sequence. The mE2-C mutants used in the experiments in (B)–(D) are shown schematically in the lower panel. Open and closed boxes indicate wild-type and mutant destruction boxes, respectively. (B) Recombinant GST fusion proteins of wild-type and mutant (Dm1 and Dm2) mE2-C were subjected to in vitro ubiquitination assay in the absence or presence of purified human APC/C. The wild-type mE2-C also was added to each reaction. The reaction mixtures were subjected to immunoprecipitation with anti-GST, and the resulting precipitates were subjected to to immunoblot analysis with antiubiquitin (left panel). The E2 activity of recombinant GST-fused wild-type and mutant (C114S, Dm1, and Dm2) proteins was examined with purified human APC/C and Myc-cyclin B as a substrate as in Figure 5A (right panel). (C) HeLa cells transfected with vectors encoding the Myc-tagged wild-type and mutant (C114S, Dm1, and Dm2) mE2-C proteins were pulse labeled with [35S]methionine and then were incubated in the absence of isotope for the indicated chase periods. Cell lysates then were subjected to immunoprecipitation with the monoclonal antibody to Myc, and the resulting precipitates were subjected to SDS-PAGE and autoradiography. (D) HeLa cells transfected with vectors encoding the Myc-tagged wild-type and mutant (Dm1 and Dm2) mE2-C proteins were arrested at metaphase by nocodazole treatment and then were incubated in nocodazole-free medium for the indicated times. Cell lysates then were subjected to immunoblot analysis with the monoclonal antibody to Myc.