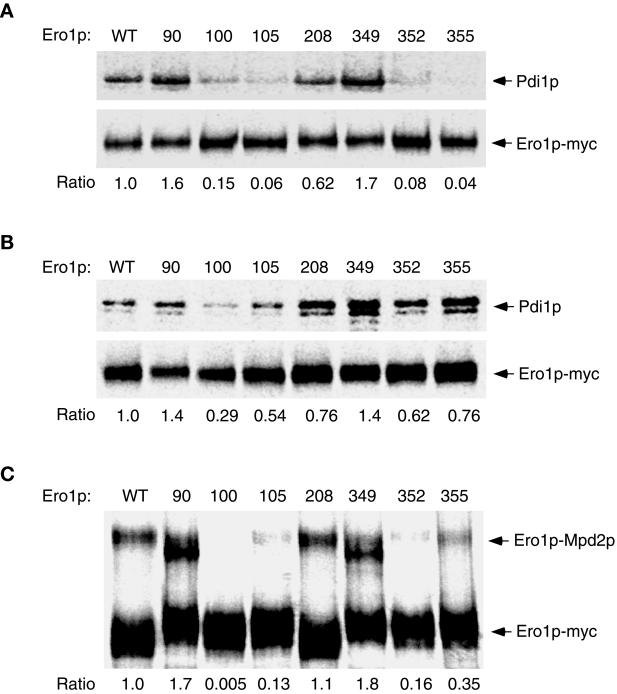
Figure 3.
The capture of mixed-disulfide complexes between each alanine substitution mutant of Ero1p-myc and CGHS–CGHS Pdi1p or CQHA Mpdp2. Cells overproducing a single alanine-substitution mutant of Ero1p-myc in addition to CGHS–CGHS Pdi1p were labeled with [35S]methionine and cysteine and then suspended in 10% TCA to block further thiol–disulfide exchange in vivo. Ero1p-Pdi1p mixed disulfides were isolated by modifying free thiols with NEM prior to immunoprecipitation with anti-myc antibody under nonreducing but denaturing conditions. The primary immunoprecipitates were reduced with 100 mM DTT prior to reimmunoprecipitation with either anti-myc (1× loading) or anti-Pdi1p (7.5× loading) antibody. Samples were resolved by SDS-PAGE and analyzed with a 445si phosphorimager. The efficiency of mixed-disulfide capture is expressed as the ratio of the band intensity of reimmunoprecipitated Pdi1p to that of reimmunoprecipitated Ero1p-myc (per OD600 U of extract), normalized to the value obtained with wild-type Ero1p-myc. (A) Strains derived from CKY598 (ero1-1 GAL2) were grown in SMM Raf/Gal and labeled at restrictive temperature (38°C). (B) Otherwise isogenic strains derived from CKY263 (GAL2) were labeled at 30°C. The strains shown were transformed with pAF132 [PGAL1-pdi1-1966] in addition to the following: WT, pAF89 [2μ ERO1-myc]; 90, pAF124 [2μ ero1-A90-myc]; 100, pAF125 [2μ ero1-A100-myc]; 105, pAF126 [2μ ero1-A105-myc]; 208, pAF127 [2μ ero1-A208-myc]; 349, pAF128 [2μ ero1-A349-myc]; 352, pAF129 [2μ ero1-A352-myc]; or 355, pAF130 [2μ ero1-A355-myc]. (C) Mixed disulfides between CQHA Mpd2p and each alanine substitution mutant of Ero1p-myc were isolated as described above from wild-type cells (CKY263) transformed with pAF123 [PGAL1-mpd2p-CQHA] instead of pAF132, and the complexes resolved directly by nonreducing SDS-PAGE. The efficiency of mixed-disulfide capture is expressed as the ratio of the band intensity of the Mpd2p-Ero1p-myc complexes to free Ero1p-myc, normalized to the value obtained with wild-type Ero1p-myc.