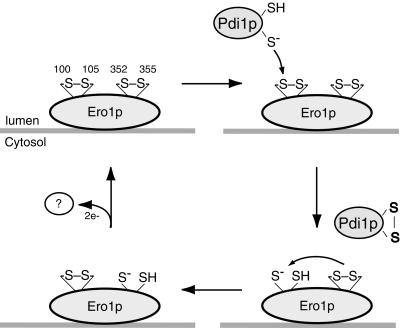
Figure 6.
Model for the catalytic mechanism of Ero1p. Two redox-active disulfide bonds are present in Ero1p (upper left). The Cys100-Cys105 disulfide engages in thiol–disulfide exchange with Pdi1p, whereas the Cys352–Cys355 disulfide serves to reoxidize the Cys100–Cys105 cysteine pair, possibly through an intramolecular thiol–disulfide exchange reaction. Reoxidation of Ero1p could proceed via oxidation of the Cys352–Cys355 cysteine pair by an as yet unidentified electron acceptor. Only one thioredoxin-like domain of Pdi1p is shown.