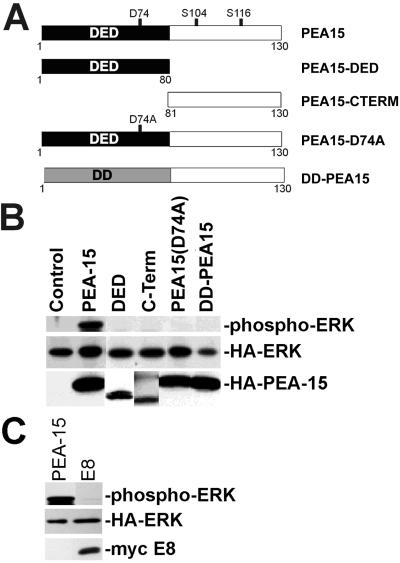
Figure 5.
The DED of PEA-15 is necessary but not sufficient for PEA-15 activation of ERK. (A) Depiction of the PEA-15 mutant constructs. The mutated aspartate (D74) is indicated, as are phoshphorylated serines (S104, S116). All recombinant proteins are fused to an HA tag. DD is structurally related to the DED. (B) αβpy-Cells were cotransfected with expression vectors encoding HA-ERK (2 μg) in combination with PEA-15 (4 μg), PEA-15-DED (DED; 8 μg), PEA-15-C-Term (C-Term; 8 μg), PEA-15(D74A) (4 μg), DD-PEA-15 (4 μg), or vector lacking insert (Control; 8 μg). Cell lysates were analyzed by SDS-PAGE followed by immunoblotting. Top, immunoblot with a polyclonal antibody specific for phosphorylated active ERK1. Note that only wild-type PEA-15 activates ERK. Middle and bottom, immunoblots with anti-HA antibody 12CA5. Note transfected HA-ERK expression levels are comparable in all transfections. Bottom, note the expression of all PEA-15 constructs. (C) αβpy-Cells were cotransfected with expression vectors encoding HA-ERK (2 μg) in combination with PEA-15 (3 μg) or E8 (3 μg). Cell lysates were analyzed by SDS-PAGE followed by immunoblotting. Top, immunoblot with a polyclonal antibody to phosphorylated ERK1/2 (phosphorylated HA-ERK is shown). Note that E8 does not activate ERK. Middle, immunoblot with anti-HA antibody. Bottom, immunoblot with antimyc antibody.