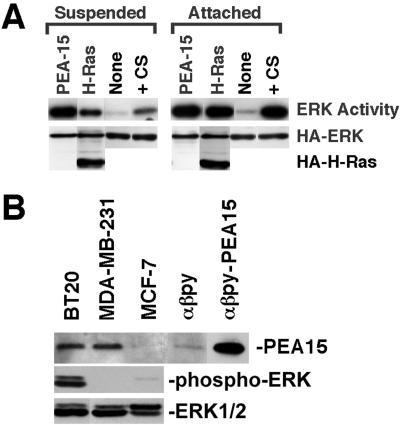
Figure 6.
PEA-15 activation of ERK2 does not require cell attachment. (A) NIH3T3 cells were cotransfected with vectors encoding HA-ERK2 (1 μg) and PEA-15 (1 μg), H-RasG12V (1 μg), or control vector lacking an insert (None; 1 μg). After 48 h, transfected cells were transferred to either agarose-coated tissue culture plates (suspended) or standard tissue culture plates (attached) and cultured an additional 24 h in low serum (0.4%). Control cells transfected with empty vector alone also were serum stimulated with 10% serum immediately before lysis (serum). Cells were lysed and recombinant ERK2 was immunoprecipitated with anti-HA antibody, 12CA5. ERK2 activity was determined by in-gel kinase assay with myelin basic protein as substrate. Top, relative ERK2 activity. Note that PEA-15 activates ERK2 to similar levels in both suspended and attached cells. Both Ras and serum stimulation are more effective in attached cells than in suspended cells. Bottom, immunoblot done with the anti-HA antibody 12CA5. Note comparable expression levels of HA-ERK and HA-tagged H-RasG12V in all transfections. (B) PEA-15 expression in mammary carcinoma cells. BT20, MDA-MB-231, MCF-7, αβpy, and PEA-15–transfected αβpy-cells were lysed in M2 buffer (under MATERIALS AND METHODS). The equivalent of 15 μg of each lysate was resolved by SDS-PAGE and transferred to a nitrocellulose membrane. PEA-15 was assayed by immunoblotting with polyclonal anti-PEA-15. ERK activity in the breast cell lines after serum starvation was measured by blotting with a phospho-ERK antibody. All blots were visualized by enhanced chemiluminescence.