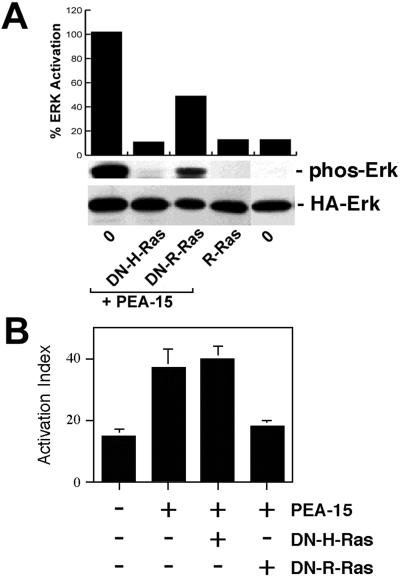
Figure 7.
PEA-15 activation of ERK is independent of its effects on integrin activation. (A) Effects on ERK pathway. CHO cells were transfected with HA-ERK2 (2 μg). They also were transfected with vectors encoding PEA-15 (3 μg) in combination with H-RasT17N (DN-H-Ras; 1 μg), R-RasT43N (DN-R-Ras; 3 μg), or pcDNA1 lacking an insert (O; 8 μg). Other cells were transfected with activated R-RasV38 (R-Ras, 3 μg). Top, cell blot was quantitated by spot densitometry and corrected for ERK levels. These data are plotted above the blot. Middle, cell lysates were immunoblotted with antibodies specific for phosphorylated ERK. Note that dominant-negative H-Ras but not dominant-negative R-Ras blocked PEA-15 activation of ERK. H-Ras and PEA-15 expression levels were similar in all transfections. R-Ras does not activate ERK in these cells. Bottom, HA-ERK2 expression levels were comparable in all transfections. (B) Effects on integrin activation. αβpy-Cells were cotransfected with Tac-α5 (2 μg) and the indicated combinations of H-RasG12V (3 μg), PEA-15 (3 μg), R-RasT43N (DN-R-Ras, 3 μg), R-RasG38V (R-Ras, 2 μg), and H-RasT17N (DN-H-Ras, 2 μg). Total amounts of transfected plasmid were adjusted to 11 μg by addition of appropriate amounts of vector lacking an insert. After 48 h, integrin activation was determined by PAC1 binding to the Tac-positive subset of cells as described (Chen et al., 1994). Depicted is the mean activation index ± SD for three independent experiments. H-Ras and PEA-15 levels were similar in all transfections.