Abstract
A 45-year-old man from Nepal with a 13-year history of polycystic kidney disease was diagnosed as suffering from chronic renal failure with end-stage renal disease. After receiving empirical antituberculosis treatment, he was treated with broad-spectrum antibiotics. A left nephrectomy was performed, and after 4 months, he received a kidney transplant. The left kidney was grossly enlarged, with multiple cystic spaces filled with blackish material. Histologic examination of the excised left kidney tissue stained with hematoxylin and eosin and Gomori's methenamine silver stains showed numerous hyaline, septate, fungal hyphae of various lengths, many broken into rectangular arthroconidia in the cystic spaces. Culture of the kidney tissue yielded white, glabrous, yeast-like colonies. Based on its micromorphology, growth at 42°C, and ribosomal DNA (rDNA) sequence analysis, and also sequence analysis of the internal-transcribed-spacer and D1/D2 rDNA regions, the yeast was identified as Trichosporon loubieri. Postsurgically, the patient was treated with amphotericin B and oral itraconazole, followed by maintenance therapy with fluconazole. He remained afebrile and asymptomatic. At the final follow-up, all parameters were found normal and the patient was doing well, with normal renal function reports. This paper presents the first known case of human infection caused by T. loubieri.
According to the new taxonomic revision of the genus Trichosporon, six species, namely, T. asahii, T. asteroides, T. cutaneum, T. inkin, T. mucoides, and T. ovoides, are recognized as medically important species causing superficial, mucosa-associated, deep-seated infections (3, 5, 7, 9, 17). The binomial name (T. beigelii) often used in the earlier literature as the causal agent of deep-seated trichosporonosis was considered to be of doubtful identity and was rejected (8). At present, T. asahii is considered the principal etiologic agent of systemic trichosporonosis and most of the clinical isolates previously identified as either T. beigelii and/or T. cutaneum from invasive deep infections, especially in immunocompromised patients, would belong to T. asahii (4, 11-16, 18, 23). We describe an infection caused by T. loubieri, a species hitherto not known to cause human infection, in a 45-year-old male from Nepal with adult polycystic kidney disease.
Case history.
A 45-year-old male resident of Katmandu, Nepal, was admitted to the Kidney Disease and Institute of Organ Transplantation department of the Madras Institute of Orthopaedics and Traumatology, Chennai, India, on 24 August 2001. The patient gave a history of polycystic kidney disease, diagnosed elsewhere 13 years prior to admission, and had suffered intermittent left-lumbar pain since then. The pain had increased in intensity during the last 6 months. Since his original diagnosis, he had received regular follow-up monitoring of urea, creatinine, and electrolytes to assess his kidney function.
At the time of his admission to the Madras Institute of Orthopaedics and Traumatology hospitals, he was diagnosed as suffering from chronic renal failure with end-stage renal disease due to adult polycystic kidney disease. The patient was moderately built but poorly nourished, conscious, febrile, and anemic (hemoglobin, 7.4 G/dl), with a pulse rate of 78 beats/min and blood pressure of 110/90. His kidney function had gradually deteriorated over the last 5 years, and his creatinine level was 4.3 mg/dl and his blood nitrogen urea level was 75 mg/dl. He was empirically treated with antituberculosis treatment for 1 month (1 to 31 August 2001). In addition, he received a total of six doses of vancomycin and amikacin (250 mg intravenously) for his fever and Bactrim double strength tablets (160 mg of trimethoprin plus 800 mg of sulfamethoxazole once daily) were given for 6 days during August and then continued from 18 December 2001 onward as prophylaxis for Pneumocystis carinii pneumonia after he had received a kidney transplant.
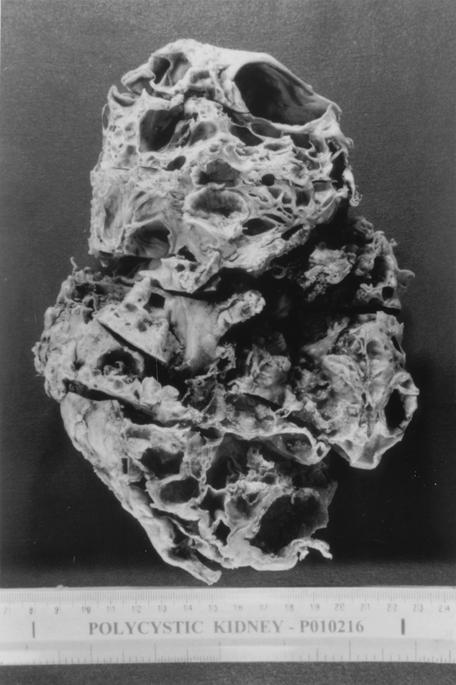
Ultrasonography of the abdomen showed both kidneys grossly enlarged, with multiple cystic lesions of various sizes. The cortex was not well defined. A calculus measuring 6 mm was seen in the upper right kidney. One of the cysts of the left kidney was filled with echogenic material consistent with polycystic renal disease, with bleeding into the left renal cyst. A left nephrectomy was performed on 31 August 2001. The kidney was grossly enlarged with multiple cystic spaces filled with blackish material (Fig. 1).
FIG. 1.
Excised left kidney showing multiple cystic spaces filled with blackish material.
Histologic examination.
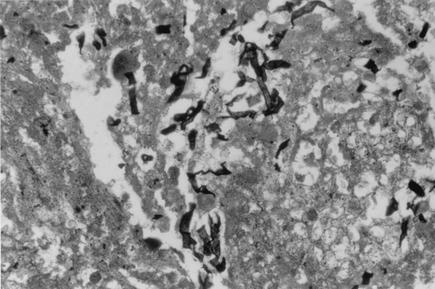
Tissue sections of the left kidney stained by hematoxylin-and-eosin and Gomori's-methenamine-silver-stain procedures showed glomeruli with sclerosis and periglomerular fibrosis. The tubules were dilated, and the cystic spaces were filled with proteinaceous fluid, blood, and cholesterol crystals. There were dense collections of neutrophils, forming abscesses in tubules and the interstitium. The blood vessels showed arteriolar sclerosis and thickening. The ureter was unremarkable. There was perirenal fat necrosis and inflammation. There were numerous hyaline, septate hyphae of various lengths, measuring 2.5 to 3.5 μm in diameter, often breaking into rectangular arthroconidia. No tissue invasion by the hyphae was observed even though the hyphal elements were seen in large numbers in the cystic spaces (Fig. 2).
FIG. 2.
Tissue slide of the excised left kidney showing septate hyphal pieces of T. loubieri from the cystic spaces without tissue invasion. Gomori's methenamine silver stain was used. Magnification, ×400.
The patient was treated with 25 mg of amphotericin B intravenously after each dialysis (16 sessions, 400 mg total). Antituberculosis treatment and all antibacterial antibiotics were discontinued after the left nephrectomy. Following surgery, the patient remained afebrile. The right nephrectomy was performed on 17 September 2001. The right kidney was also grossly enlarged and had multiple cystic spaces, some of which were filled with blackish material that was probably clotted blood. The specimens collected from the right kidney were determined to be negative for fungi both by histology (hematoxylin-and-eosin-stained sections) and by culture. The patient received an additional 50 mg of intravenous amphotericin B after each dialysis (16 sessions, 800 mg total). In addition, the patient received itraconazole orally (100 mg once daily) for 6 weeks. He received a kidney transplant on 18 December 2001. Starting from 18 December 2001, the patient received fluconazole (50 mg once daily) for a period of 3 months. He remained afebrile and asymptomatic. The final follow-up was done on 3 March 2002. All parameters were found normal, and the patient was doing well, with normal renal function reports posttransplantation.
Laboratory findings.
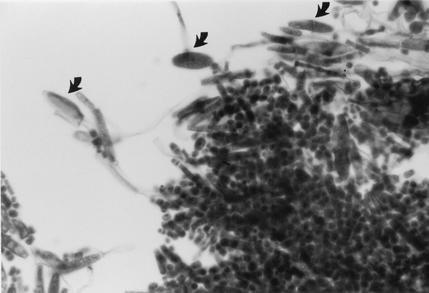
A portion of the tissue from the left kidney was cultured on Sabouraud dextrose agar containing chloramphenicol and Sabouraud dextrose agar containing chloramphenicol and cycloheximide. Cultures were incubated at 25 and 37°C. After 1 week of incubation, many white to off-white, dry, yeast-like colonies grew at both temperatures of incubation. Two single colonies showing slight morphological variation were selected for further studies (Centers for Disease Control and Prevention strains B-6228 and B-6229). Both variants were urease positive, grew at 37 and 42°C, and tolerated 0.1% cycloheximide. When tested with the API 20C AUX yeast identification kit (bioMérieux, Hazelwood, Mo.), no definitive identification could be obtained because the species was not in the API profile index (for B-6228, API 20C AUX 6777776 [ID 32C 7777676265]; for B-6229, API 20C AUX 6777775 [ID 32C 7757747677]). Microscopically, both variants on cornmeal Tween 80 agar formed true hyphae disarticulating into rectangular arthroconidia and one- or two-celled, fusiform, giant cells (10) (Fig. 3). However, on repeated subcultures, both variants failed to produce fusiform cells consistently. Both variants, based on their morphological features, were provisionally identified as T. loubieri. Subcultures were sent to the Clinical Microbiology Section, Mayo Clinic, Rochester, Minn., for further testing.
FIG. 3.
T. loubieri on cornmeal agar showing hyphae breaking into rectangular arthroconidia and one- or two-celled fusiform giant cells (arrows). Magnification, ×400.
MicroSeq D2 large-subunit ribosomal DNA (rDNA) fungal identification (Applied Biosystems, Foster City, Calif.) was performed as follows. Briefly, one loopful of growth (i.e., enough to fill the eye of a 1-μl loop) was inoculated into a tube containing 200 μl of sample preparation reagent (PrepMan Ultra sample preparation reagent [Applied Biosystems]). The samples were boiled for 10 min and centrifuged, and a 1:50 dilution was used for amplification with the use of the MicroSeq D2 large-subunit rDNA fungal sequencing kit (Applied Biosystems). The amplified product was purified by adding 1 μl each of shrimp alkaline phosphatase and exonuclease (USB Corp.) to 9 μl of PCR product. The mixture was incubated at 37°C for 30 min and then at 80°C for 15 min. Cycle sequencing was performed with the sequencing module and the conditions recommended by the manufacturer on an ABI Prism 3100 genetic analyzer (Applied Biosystems). Data analysis was done with MicroSeq, version 2000 (Applied Biosystems), and the D2 library of rDNA sequences, version 0050 (D2). The sequences of both colony types were compared with those of 1,069 entries in D2, version 0050, and those of both isolates perfectly matched the sequence of T. loubieri.
Both isolates were sent to Jack W. Fell, Rosenstiel School of Marine and Atmospheric Science, University of Miami, Key Biscayne, Fla., for confirmation of our identification. Based on rDNA sequence analysis of the internal-transcribed-spacer and D1/D2 rDNA regions (GenBank accession number AF075522), both isolates were confirmed as T. loubieri (6).
T. loubieri can be distinguished from other species of Trichosporon by its ability to grow at 42°C, assimilation of l-rhamnose, and production of lateral or intercalary, large, fusiform cells with granular contents (10). T. loubieri has been isolated previously from soil polluted with diesel fuel in The Netherlands, from the milk of a cow with mastitis in Australia, from milk in the United States, and from a sewage treatment plant in Japan (2). To our knowledge, the present case represents the first human infection caused by this species.
Trichosporonosis due to T. asahii (T. beigelii) in immunocompromised patients, those undergoing therapy for hematologic malignancies, may cause fatal infections because many strains may not be killed by amphotericin B at achievable concentrations in serum (19-21). T. asahii (T. beigelii) has also been found to be resistant to the fungicidal effects of liposomal amphotericin B (22). The antifungal triazoles fluconazole and SCH 39304 were found to be the most active against disseminated Trichosporon infections (1, 22). The usefulness of itraconazole in trichosporonosis is not well known (11). In the present case, nephrectomy of the left kidney was instrumental in removing the infection in totum. Postsurgical treatment with subtherapeutic dosages of amphotericin B, itraconazole, and fluconazole led to uncomplicated recovery of the patient without any relapse of the infection.
Acknowledgments
We thank Juliette Morgan, Division of Bacterial and Mycotic Diseases, Centers for Disease Control and Prevention, for her valuable suggestions concerning the clinical history and David Pincus, bio-Ḿerieux, Inc., and Jack W. Fell, University of Miami, for confirming the identities of the two isolates.
REFERENCES
- 1.Anaissie, E., A. Gokaslan, R. Hachem, R. Rubin, G. Griffin, R. Robinson, J. Sobel, and G. Bodey. 1992. Azole therapy for trichosporonosis: clinical evaluation of eight patients, experimental therapy for murine infection, and review. Clin. Infect. Dis. 15:781-787. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, J. A., R. W. Payne, and D. Yarrow. 2000. Yeast characteristics and identification, 3rd ed., p. 765. Cambridge University Press, Cambridge, United Kingdom.
- 3.Blanchard, A., F. Gouin, S. Dunan, and M. Quilici. 1995. Infection urinaire a Trichosporon inkin. Med. Maladies Infect. 25:605-606. [Google Scholar]
- 4.Chakrabarti, A., R. K. Marhawa, R. Mondal, A. Trehan, S. Gupta, D. S. V. Raman Rao, S. Sethi, and A. A. Padhye. 2001. Generalized lymphadenopathy caused by Trichosporon asahii in a patient with Job's syndrome. Med. Mycol. 40:83-86. [DOI] [PubMed] [Google Scholar]
- 5.Febre, N., V. Silva, E. A. S. Medeiros, S. B. Wey, A. L. Colombo, and O. Fischman. 1999. Microbiological characteristics of yeasts isolated from urinary tracts of intensive care unit patients undergoing urinary catheterization. J. Clin. Microbiol. 37:1584-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fell, J. W., H. Roeijmans, and T. Boekhout. 1999. Cystofilobasidiales, a new order of basidiomycetous yeasts. Int. J. Syst. Bacteriol. 49:907-913. [DOI] [PubMed] [Google Scholar]
- 7.Gueho, E., M. T. Smith, G. S. de Hoog, G. Billon-Grand, R. Christen, and W. H. Batenburg-van der Vegte. 1992. Contributions to a revision of the genus Trichosporon. Antonie Leeuwenhoek 61:289-316. [DOI] [PubMed] [Google Scholar]
- 8.Gueho, E., G. S. de Hoog, and M. T. Smith. 1992. Neotypification of the genus Trichosporon. Antonie Leeuwenhoek 61:285-288. [DOI] [PubMed] [Google Scholar]
- 9.Gueho, E., L. Improvisi, G. S. de Hoog, and B. Dupont. 1994. Trichosporon on humans: a practical account. Mycoses 37:3-10. [DOI] [PubMed] [Google Scholar]
- 10.Gueho, E., M. T. Smith, and G. S. de Hoog. 1998. Trichosporon behrend, p. 854-892, In C. P. Kurtzman and J. W. Fell (ed.), The yeasts: a taxonomic study. Elsevier Science B. V., Amsterdam, The Netherlands.
- 11.Itoh, T., H. Hosokawa, U. Kohdera, N. Toyazaki, and Y. Asada. 1995. Disseminated infection with Trichosporon asahii. Mycoses 39:195-199. [DOI] [PubMed] [Google Scholar]
- 12.Lopes, J. O., S. H. Alves, C. Klock, L. T. O. Oliveira, and N. R. F. Dal Forno. 1997. Trichosporon inkin peritonitis during continuous ambulatory peritoneal dialysis with bibliography review. Mycopathologia 139:15-18. [DOI] [PubMed] [Google Scholar]
- 13.Mahal, M., L. Saiman, L. Bitman, I. Weitzman, M. Grossman, F. Dembitzer, P. Della-Latta, and J. Garvin. 1998. Review of trichosporonosis with a report of a case of disseminated Trichosporon asahii infection. Infect. Dis. Clin. Pract. 7:175-179. [Google Scholar]
- 14.Melez, K. A., J. Cherry, C. Sanchez, R. B. Ettinger, and T. J. Walsh. 1995. Successful outpatient treatment of Trichosporon beigelii peritonitis with oral fluconazole. Pediatr. Infect. Dis. J. 14:1110-1113. [PubMed] [Google Scholar]
- 15.Mirza, S. H. 1993. Disseminated Trichosporon beigelii infection causing skin lesions in a renal transplant patient. J. Infect. 27:67-70. [DOI] [PubMed] [Google Scholar]
- 16.Nagappan, R., J. F. Collins, and W. T. Lee. 1992. Fungal peritonitis in continuous ambulatory peritoneal dialysis—the Auckland experience. Am. J. Kidney Dis. 20:492-496. [DOI] [PubMed] [Google Scholar]
- 17.Sugita, T., A. Nishikawa, R. Ikeda, and T. Shinoda. 1999. Identification of medically relevant Trichosporon species based on sequences of internal transcribed spacer regions and construction of a database for Trichosporon identification. J. Clin. Microbiol. 37:1985-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takamura, S., T. Oono, H. Kauzaki, and J. Arata. 1999. Disseminated trichosporonosis with Trichosporon asahii. Eur. J. Dermatol. 9:577-579. [PubMed] [Google Scholar]
- 19.Walsh, T. J. 1989. Trichosporonosis. Infect. Dis. Clin. N. Am. 3:43-52. [PubMed] [Google Scholar]
- 20.Walsh, T. J., K. R. Newman, M. Moody, R. C. Wharton, and J. C. Wade. 1986. Trichosporonosis in patients with neoplastic disease. Medicine 65:268-279. [DOI] [PubMed] [Google Scholar]
- 21.Walsh, T. J., G. P. Melcher, M. G. Rinaldi, J. Lecciones, D. A. McGough, P. Kelley, J. Lee, D. Callender, M. Rubin, and P. A. Pizzo. 1990. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J. Clin. Microbiol. 28:1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh, T. J., J. W. Lee, G. P. Melcher, E. Navarro, J. Bacher, D. Callender, K. D. Reed, T. Wu, G. Lopez-Berestein, and P. A. Pizzo. 1992. Experimental Trichosporon infection in persistently granulocytopenic rabbits: implications for pathogenesis, diagnosis, and treatment of an emerging opportunistic mycosis. J. Infect. Dis. 166:121-133. [DOI] [PubMed] [Google Scholar]
- 23.Wang, H. Y., and J. L. Lin. 1999. Trichosporon beigelii fungemia in a patient with haemodialysis. Nephrol. Dialysis Transplant. 14:2017-2018. [DOI] [PubMed] [Google Scholar]