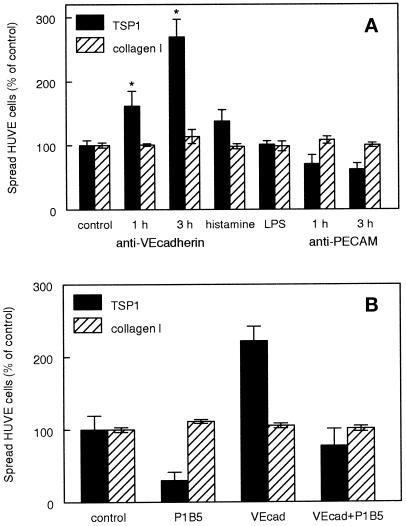
Figure 5.
Activation of endothelial cell α3β1 integrin is regulated by disruption of VE-cadherin. (A) Inhibition of VE-cadherin binding specifically induces spreading on TSP1 of confluent HUVE cell cultures. Confluent HUVE cell cultures were treated for 1 or 3 h with 5 μg/ml function-blocking VE-cadherin antibody clone 75, for 3 h with 3 μM histamine, 10 ng/ml lipopolysaccharide (LPS), or for 1 or 3 h with 5 μg/ml function-blocking PECAM-1 antibody HEC7. The cells were then dissociated with the use of EDTA and spreading was determined on substrates coated with 40 μg/ml TSP1 (closed bars) or 5 μg/ml type I collagen (striped bars). Results for both proteins are presented as percent of the spreading determined for control confluent cultures treated for 3 h with the medium alone (mean ± SD, n = 3). (B) α3β1 integrin mediates endothelial cell spreading stimulated by disrupting VE-cadherin. Confluent HUVE cells were mock treated or treated for 3 h with 5 μg/ml function-blocking VE-cadherin antibody clone 75. The cells were harvested, and spreading on TSP1 or type I collagen was determined in the presence or absence of the α3β1 integrin-blocking antibody P1B5.