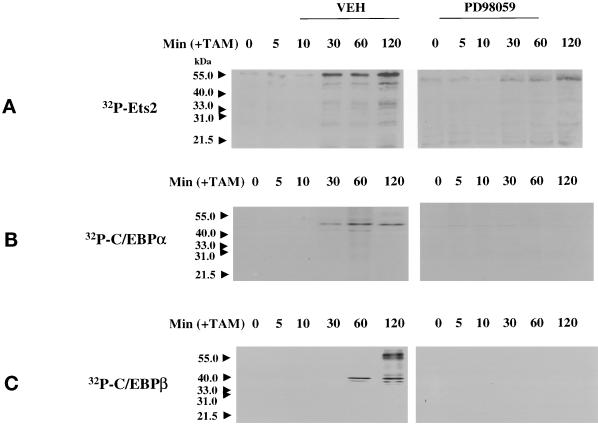
Figure 6.
MAPK signaling increases the phosphorylation of Ets2, C/EBPα, and C/EBPβ in primary hepatocytes. (A) MAPK activation increases the phosphorylation of Ets2. (B) MAPK activation increases the phosphorylation of C/EBPα. (C) MAPK activation increases the phosphorylation of C/EBPβ. Hepatocytes were infected with kinase active ΔB-Raf:ER poly-L-lysine adenovirus (250 m.o.i.), followed by culture as in Methods. After 24 h to allow protein expression, hepatocytes were treated with either vehicle control or with 100 nM 4-hydroxytamoxifen (TAM), and with or without 50 μM PD98059, for 0 to 120 min at 37°C. In experiments using 32P-labeling of cells, phosphate free media was used and cells were preincubated with 32P for 2 h before the start of 4-hydroxytamoxifen treatment. No significant alteration in the immunoblotting amounts of Ets2, C/EBPα, or C/EBPβ occurred during the 2-h time course of the experiment (our unpublished data). The MAPK-dependent incorporation of 32P into either Ets2, C/EBPα, or C/EBPβ was determined following immunoprecipitation, SDS PAGE, and transfer to nitrocellulose. Incorporation of 32P into Ets2 and C/EBP proteins was determined after immunoblotting by autoradiography.