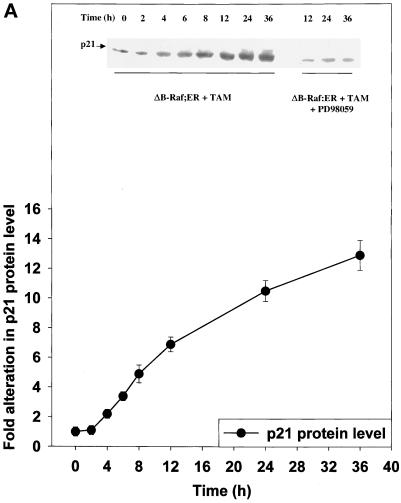
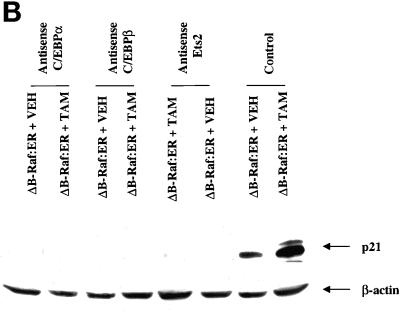
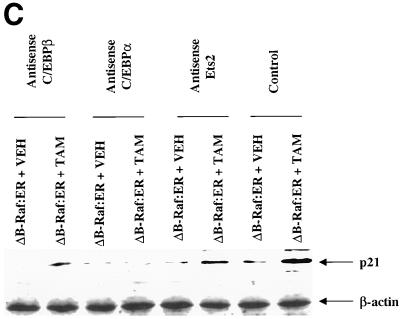
Figure 7.
MAPK signaling increases p21 protein levels via multiple transcription factors. (A) Time course and quantitation of p21 protein induction by ΔB-Raf:ER. (B) Expression of antisense C/EBPα, antisense C/EBPβ, and antisense Ets2 modify both basal and ΔB-Raf:ER-mediated p21 protein induction 8 h after MAPK activation. (C) Expression of antisense C/EBPα, antisense C/EBPβ, and antisense Ets2 modify both basal and ΔB-Raf:ER-mediated p21 protein induction 36 h after MAPK activation. Hepatocytes were infected with kinase active ΔB-Raf:ER poly-L-lysine adenovirus (250 m.o.i.), followed by culture as in Methods. In some experiments and in addition to ΔB-Raf:ER infection, cells were also infected with either an additional null plasmid poly-L-lysine adenovirus or with either antisense C/EBPα or antisense C/EBPβ poly-L-lysine adenoviruses (at 200 m.o.i. each). In some experiments and in addition to ΔB-Raf:ER infection, cells were also transfected with antisense oligonucleotides toward Ets2. Transfection with a scrambled antisense Ets2 oligonucleotide did not alter basal or stimulated p21 protein levels (our unpublished observations). After 24 h to allow protein expression, hepatocytes were treated with either vehicle control or with 100 nM 4-hydroxytamoxifen for either 8 h or 36 h. Protein expression of p21 was determined by immunoblotting. Equal protein loading (Panels A and C: 200 μg. Panel B: 400 μg) per lane; exposures of x-ray film for the ECL immunoblots shown were from 30 s to 1 min. (A) Time course 0 to 36 h after either ΔB-Raf:ER + 4-hydroxytamoxifen or ΔB-Raf:ER + 4-hydroxytamoxifen + 50 μM PD98059. (B and C) Lanes with either ΔB-Raf:ER + vehicle control (VEH) or ΔB-Raf:ER + 4-hydroxytamoxifen (TAM). Equal protein amounts were loaded, and the internal control immunoblot was β-actin. A representative experiment for each cell type/condition is shown (panel B: n = 3. panel C: n = 6).