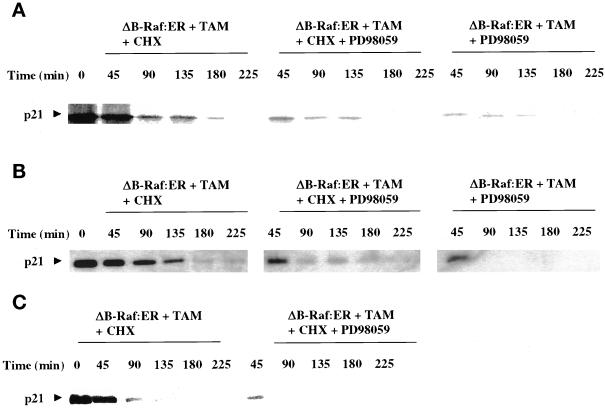
Figure 9.
MAPK activation increases the protein stability of p21 in primary hepatocytes and HepG2 cells. Primary hepatocytes (panels A and B) and HepG2 cells (panel C) were infected with kinase active ΔB-Raf:ER poly-L-lysine adenovirus (250 m.o.i.), followed by culture as in Methods. (A and C) After 24 h to allow ΔB-Raf:ER protein expression, cells were incubated in methionine/cysteine-free culture media containing 0.1 μCi/10 μl [35S]methionine. Cells were treated for 480 min with 4-hydroxytamoxifen. After 480 min, [35S]methionine-containing media was replaced with media containing methionine/cysteine, still containing 4-hydroxytamoxifen, and where indicated including MAPK (50 μM PD98059)/translation inhibitors (20 μg/ml cycloheximide). (B) After 24 h to allow ΔB-Raf:ER protein expression, cells were treated for 32 h with 4-hydroxytamoxifen, and then incubated in methionine/cysteine-free culture media for a further 4 h containing 0.1 μCi/10 μl [35S]methionine and 4-hydroxytamoxifen. After 36 h (total time), [35S]methionine-containing media was replaced with media containing methionine/cysteine, still containing 4-hydroxytamoxifen, and where indicated including translation inhibitor. The incorporation of [35S]methionine into p21 protein was determined following p21 immunoprecipitation over a time course following removal of [35S]methionine. Equal protein loading (200 μg) per lane; p21 protein was detected by autoradiography of radiolabeled p21-bands and immunoblotting. Exposures of x-ray film for immunoblots shown were 30 s to 1 min. A representative experiment for each condition is shown (n = 3).