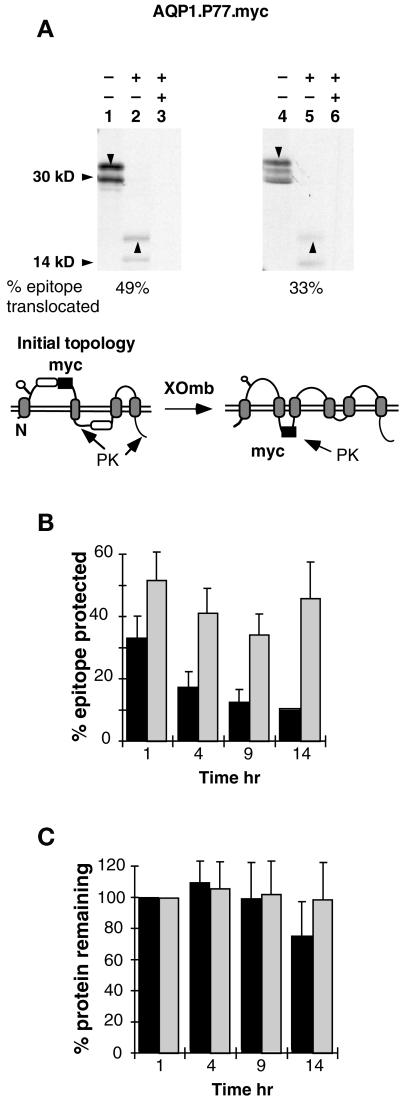
Figure 7.
Topological reorientation of TM3 N-terminal flanking residues. (A) AQP1.P77.myc expressed in RRL supplemented with CRM (lanes 1–3) or XOmb (lanes 4–6) was digested with PK and immunoprecipitated with anti-myc antibody as in Figure 4. Downward arrowheads indicate full-length core-glycosylated AQP1 constructs. Upward arrowheads indicate glycosylated myc-reactive fragments derived from full-length polypeptides. The diagram shows the orientation of the myc epitope that would give rise to the protected fragment(s). (B) Pulse-chase analysis of AQP1.P77.myc expressed in RRL supplemented with CRM (hatched bars) or XOmb (solid bars) was performed as in Figure 5. The percent epitope protected represents the fraction of full-length glycosylated chains that gave rise to the 19-kDa myc-reactive, PK-protected fragments. Translocation values are corrected for methionine content and represent the average of six (CRM) or two to four (XOmb) experiments. (C) Total protein was averaged from seven (CRM) or five (XOmb) experiments and normalized for protein present at 1 h (± SE).