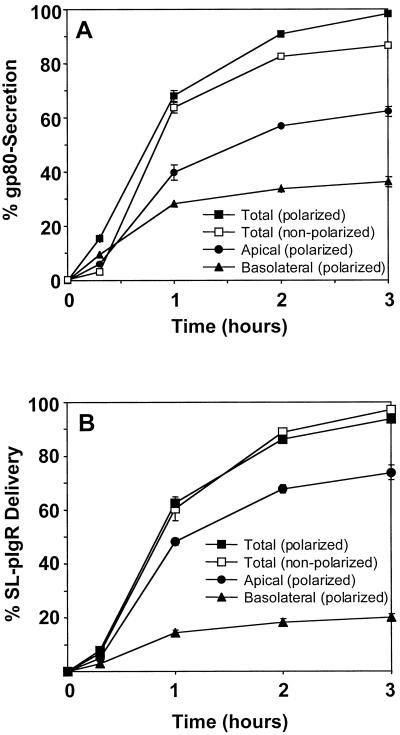
Figure 10.
Quantitation of surface transport of the secreted protein gp80/clusterin in polarized and nonpolarized MDCK cells. MDCK cells (A) or MDCK cells stably expressing SL-pIgR (B) were grown as either a polarized monolayer on polycarbonate filters in regular medium or as nonpolarized cells on glass coverslips in LCM. Proteins were pulse-labeled with [35S]cysteine. (A) The secretion of radiolabeled gp80 was monitored by collecting apical or basolateral medium (polarized) or total medium (nonpolarized) at different times and quantitation by SDS-PAGE and phosphorimaging and is represented as a percentage of total radiolabeled gp80 (intracellular plus secreted). Apically and basolaterally secreted gp80 was added to yield total secretion of gp80 from polarized cells. Note that after 3 h, gp80 secretion was nearly complete in polarized cells, whereas ∼15% of gp80 remained intracellular in nonpolarized cells. (B) The surface delivery of newly synthesized SL-pIgR was monitored by the addition of a low concentration of V8 protease to the chase medium, which efficiently released the extracytoplasmic domain of pIgR into the medium. Quantitation was as described above. Note that the kinetics of SL-pIgR delivery in polarized and nonpolarized cells is nearly identical, indicating that the majority of SL-pIgR is delivered directly to the plasma membrane. Experiments were done in triplicate, and error bars represent SDs. Error bars were omitted when they were smaller than the symbol.