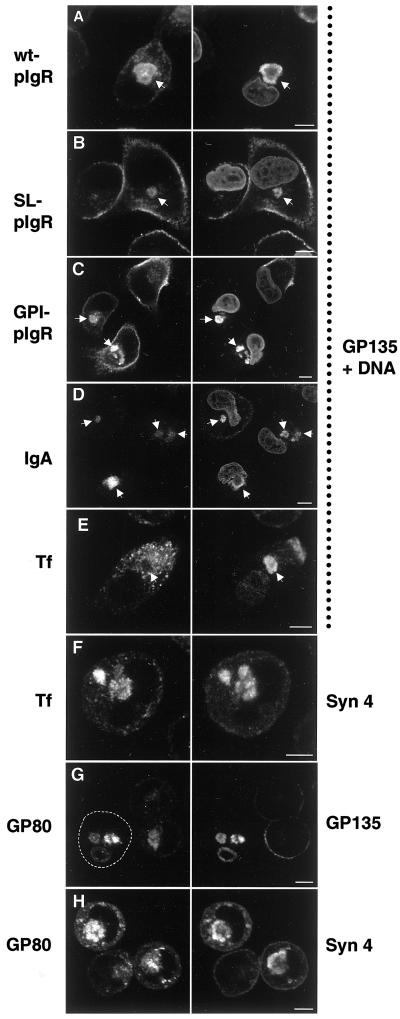
Figure 9.
The “apical” and “basolateral” intracellular compartments receive membrane traffic from cognate polarized trafficking pathways. MDCK cells or MDCK cells stably expressing the wild-type pIgR (wt-pIgR), signalless pIgR (SL-pIgR), or a GPI-anchored mutant form of pIgR (GPI-pIgR) were grown in LCM for 16 h. IgA, 50 μg/ml polymeric IgA was added during the 16-h incubation of cells expressing wild-type pIgR. Tf, 1 μg/ml iron-loaded caninetransferrin was added to MDCK cells during the incubation. The cells were fixed, permeabilized, and stained with an antibody against the endogenous apical plasma membrane protein gp135 to label VACs and propidium iodide as a nuclear stain (right column, as indicated). The pIgR was detected with the use of an antibody against the ectodomain (left column, top three panels). IgA, transferrin, syntaxin 4, and gp80/clusterin were detected with the use of specific antibodies. Note that wt-pIgR, SL-pIgR, and GPI-pIgR are transported to the VAC (arrows). Also, IgA added to the medium of wt-pIgR–expressing cells is transported to the VAC (arrows), indicating that wt-pIgR is first transported to the plasma membrane after biosynthesis, where it can bind IgA, internalize it, and transport it into the VAC. In contrast, internalized transferrin does not reach the VAC (arrow). Instead, it is deposited into a syntaxin 4–positive compartment (F). The endogenous soluble protein gp80/clusterin, which is normally secreted apically and basolaterally (∼2:1 ratio) in polarized MDCK cells, is transported to both VACs (G) and syntaxin 4–positive organelles (H) in nonpolarized cells, suggesting that it reaches these compartments directly after biosynthesis. The panels in the left column show the same fields as those in the right column. Arrows are drawn for better orientation. The outline of one cell in G is drawn for clarity. Bars, 5 μm.