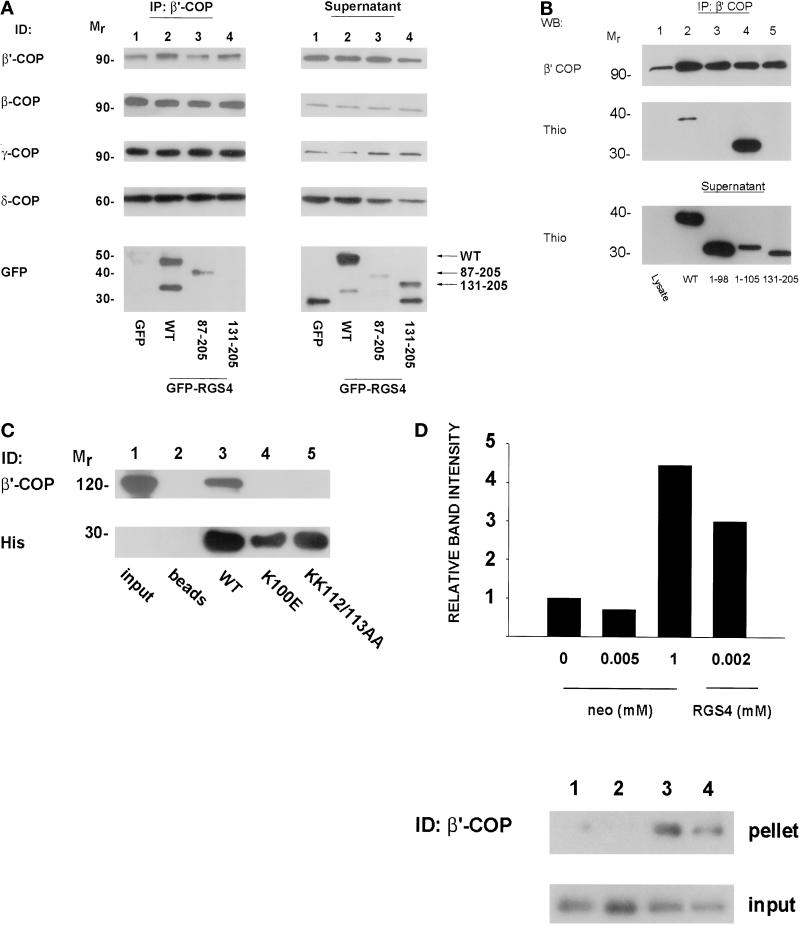
Figure 2.
(A) Coimmunoprecipitation of RGS4 and coatomer from HEK293TT cells. GFP–RGS4 full-length and deletion (87–205 and 131–205) fusion proteins were expressed in HEK293TT cells and immunoprecipitated with antibody to β′-COP as described in MATERIALS AND METHODS. The entire amount of each immunoprecipitation was separated on SDS gels, transferred to nitrocellulose membranes, and blotted with antibodies against GFP or β-, β′-, γ-, or δ-COP as shown in the left panel (IP: β′-COP). Equal amounts of protein from postimmunoprecipitation lysates (quantitated by Bradford assay) were then separated by SDS-PAGE and immunodetected (ID) as labeled (Supernatant). (B) Mapping of the β′-COP binding region of RGS4. Truncated RGS4 proteins fused to thioredoxin were expressed and purified from E. coli. Coatomer from rat liver cytosol was immunoprecipitated with anti-β′-COP antibody, and immunoprecipitates were then incubated with thioredoxin fusion proteins. After washing away unbound proteins, each immunoprecipitate was electrophoresed and immunodetected as labeled. Postimmunoprecipitation supernatants (bottom panel) were incubated with Thio-bond resin, washed, fractionated, and immunoblotted with an anti-thioredoxin antibody to ensure that the amounts of thioredoxin fusion protein added were not limiting. (C) Mapping of RGS4 residues involved in β′-COP binding. Hexahistidine-tagged wild-type or mutant RGS4 proteins (5 μg) were incubated with nickel beads and GST–β′-COP (2 μg) and processed as in Figure 1B. (D) Relative affinity of COPI for RGS4 and neomycin. Rat liver cytosol was incubated with varying concentrations of either neomycin sulfate or 6His-RGS4 before centrifugation at 100,000 × g. Supernatants were removed, and the pellets were solubilized by boiling in Laemmli buffer before electrophoresis on SDS gels. The relative amounts of precipitated β′-COP in the pellet were assessed by immunoblotting (top panel). An equal amount of supernatant was removed before centrifugation to assess the amount of input protein for each condition (bottom panel). The bar graph represents the relative amount of β′-COP precipitated in each condition for the immunoblot shown as measured by densitometry. Similar results were observed in three independent experiments.