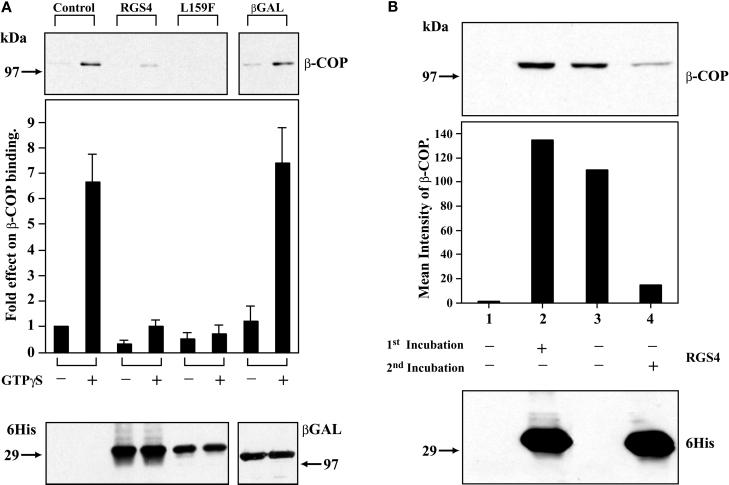
Figure 4.
Inhibition of COPI binding to Golgi membranes by RGS4. (A) Both purified, wild-type RGS4 and RGS4(L159F) inhibit binding of COPI to Golgi membranes in vitro. Golgi membranes were incubated with equal amounts of 6His-RGS4 (wild type), 6His-RGS4 (L159F), or 6His-β-Gal (5 μg) in the presence of rat liver cytosol (1 mg of protein). After extensive washing, membranes were solubilized in Laemmli buffer, electrophoresed on SDS gels, and immunoblotted for bound β-COP (top panel). Before washing, aliquots of the total mixture (membranes plus cytosol) were removed, electrophoresed, and immunoblotted with an anti-His antibody (bottom panel). The bar graph represents means ± SEM of bound β-COP intensity under various conditions (five independent experiments). (B) Membrane-bound RGS4 does not inhibit β-COP Golgi membrane binding. 6His-RGS4 was incubated with Golgi membranes before addition of cytosol (bottom panel, lane 2); all other lanes contained only buffer in the first incubation. Membranes were pelleted by centrifugation, and unbound protein was removed by washing. Pellets were then incubated with cytosol, GTPγS (25 μM), and either buffer (lane 2) or 6His-RGS4 (lane 4) in a second incubation before recentrifugation, solubilization in Laemmli buffer, and electrophoresis and immunoblotting with a β-COP antibody (top panel). The bar graph represents the relative β-COP intensity for each condition in the experiment shown; a subsequent similar experiment gave equivalent results.