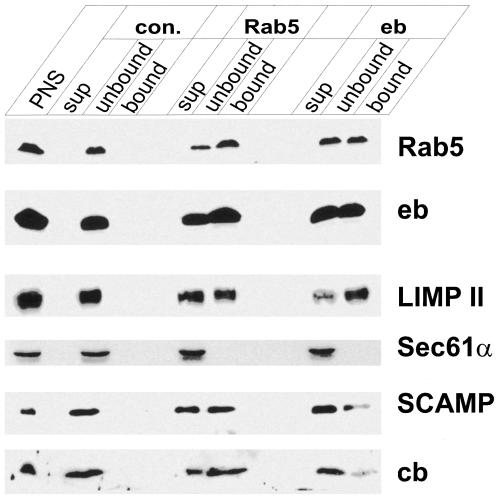
Figure 3.
Characterization of organelles immunoisolated with microbeads containing antibodies specific for endobrevin and Rab5. A PNS from rat liver was incubated with small amounts of immunobeads containing covalently bound antibodies specific for Rab5 and endobrevin (eb). Glycine-inactivated beads (con.) served as control for nonspecific binding. The protein composition of the bead-bound membranes (bound) was compared with that of unbound membranes that were sedimented by ultracentrifugation (unbound). Equal proportions of all fractions were analyzed by immunoblotting. PNS, postnuclear supernatant used as starting material; sup, membrane-free supernatant obtained after ultracentrifugation and concentrated by precipitation according to Wessel and Flügge (1984); cb, cellubrevin. PNS, supernatant, and unbound fractions, 10 μg of protein/lane; bead-bound material, ∼6 μl of beads. Note that the membrane protein content of the beads is at least 5- to 10-fold lower than of the unbound fraction. Also, note that to saturate the beads with organelles, an excess of PNS was used, explaining why none of the antigens was depleted.