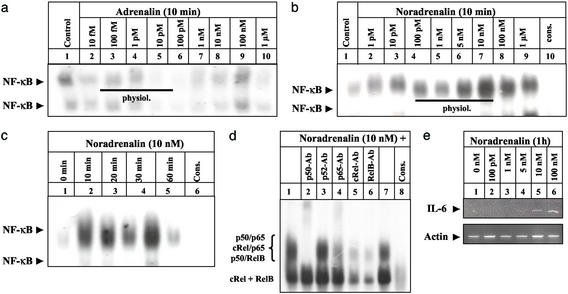
Figure 3.
Physiological concentrations of NA induce functionally significant NF-κB-binding activity in cultured THP-1 monocytic cells. (a and b) THP-1 cells were left untreated (lane 1) or incubated with increasing concentrations of AD (a) and NA (b) for 10 min (lanes 2–9) before NF-κB-binding activity was monitored. NF-κB binding was confirmed by competing with a 160-fold molar excess of unlabeled NF-κB consensus oligonucleotides (b; cons, lane 10). A black line indicates the physiological concentration range of AD and NA. The experiment was repeated two times with identical results and one representative experiment is shown. (c) THP-1 cells were either left untreated (0 h) or stimulated with NA (10 nM) for 10 min to 1 h (lanes 2- 5), and nuclear extracts were assayed for NF-κB-binding activity as above. The experiment was performed twice with identical results and one representative experiment is shown. (d) Characterization of the NF-κB subunits contributing to the NA-induced-binding activity at the NF-κB consensus sequence (lanes 1 and 7) was performed by including 2.5 μg of anti-p50 (lane 2), anti-p52 (lane 3), anti-p65 (lane 4), anti-cRel (lane 5), or anti-relB (lane 6) Abs in the binding reaction. Specificity of NF-κB binding was confirmed as above (lane 10). The position of the different NF-κB complexes formed is indicated at the left. (e) THP-1 cells were either left untreated (0 h) or stimulated with different concentrations of NA (lanes 2–6) for 1 h. Total RNA was prepared and analyzed by RT-PCR with primers specific for human IL-6 and β-actin, respectively.