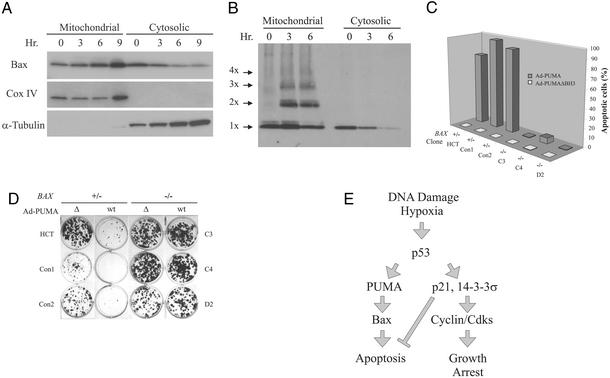
Figure 5.
BAX deficiency rescues PUMA-induced apoptosis. (A) Mitochondrial and cytosolic fractions were prepared from DLD-1 cells in which PUMA had been induced by the removal of doxycycline. The fractions were analyzed by immunoblotting with antibodies to the indicated proteins. Cox IV and α-tubulin provided controls for the fractionation. (B) DLD-1 cells in which PUMA had been induced were cross-linked before subcellular fractionation and subjected to immunoblotting with an antibody to Bax. Bax monomers (1X) and multimers (2X–4X) are indicated. (C) Clones with various BAX genotypes were infected with Ad-PUMA (wt) or Ad-PUMAΔBH3 (Δ) for 48 h and the fraction of apoptosis was assessed by fluorescence microscopy of DAPI-stained cells. (D) HCT116 clones with the indicated BAX genotypes were infected with Ad-PUMA (wt) or Ad-PUMAΔBH3 (Δ) for 48 h then diluted into 12-well plates. Colonies were stained with crystal violet 10–14 days later. (E) Model of p53 function during tumorigenesis. As tumors progress to malignancy, they accumulate DNA damage (1). They also outgrow their blood supply, resulting in hypoxia of large regions of the tumors (31). The DNA damage and/or hypoxia activate p53, which transcriptionally activates several genes, including p21, 14-3-3σ, PUMA, and, in some cases, BAX. The cells either arrest or enter into apoptosis, depending on which of the two arms of the pathway is predominant in the particular cell type and microenvironment. Cells with a mutant p53 gene would have a selective growth advantage in either case, because both growth arrest and apoptosis would be diminished in such mutant cells. Although many other genes are induced by p53 (7), the ones included in the model have all been shown to be essential for the indicated downstream effects through gene-targeting experiments in mammalian cells.