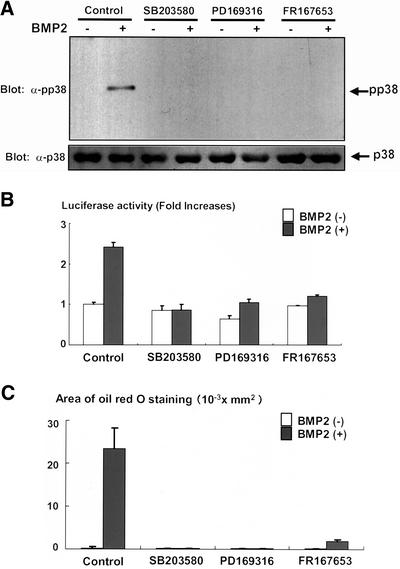
Figure 7.
(A) Inhibition of p38 kinase activation by PD169316 and FR167653. C3H10T1/2 cells were serum starved in 0.2% fetal calf serum-DMEM for 12 h. The cells were further incubated with or without p38 kinase inhibitor SB203580 (10 μM), PD169316 (1 μM), or FR167653 (10 μM) for 4 h. The cells were then stimulated with BMP2 (300 ng/ml) for 10 min and lysed, and the cell lysates were determined by immunoblotting with anti-phospho-p38 kinase (top) and anti-p38 kinase antibodies (bottom). (B) PD169316 and FR167653 inhibited transcriptional activity of PPARγ induced by BMP2. C3H10T1/2 cells were transfected together with PPARγ (0.1 μg) and RXR (0.1 μg) expression vectors and PPRE-Luc (0.1 μg) and TK-renilla (0.1 μg) reporter constructs, and pcDNA3 (1.6 μg). Twelve hours after transfection, cells were incubated with or without SB203580 (10 μM), PD169316 (1 μM), or FR167653 (10 μM) for 2 d. At the end of the culture, cells were lysed and the luciferase activity was measured and normalized by determining renilla luciferase activity as described in the text. Data were shown as mean ± SD. (C) PD169316 and FR167653 inhibited BMP2-induced adipogenesis. C3H10T1/2 cells were incubated with or without BMP2 (300 ng/ml), in the presence or absence of SB203580 (10 μM), PD169316 (1 μM), or FR167653 (10 μM) for 10 d. The cells were stained with Oil Red O, photographed under a microscope, and the areas stained with Oil Red O in the cells were assessed.