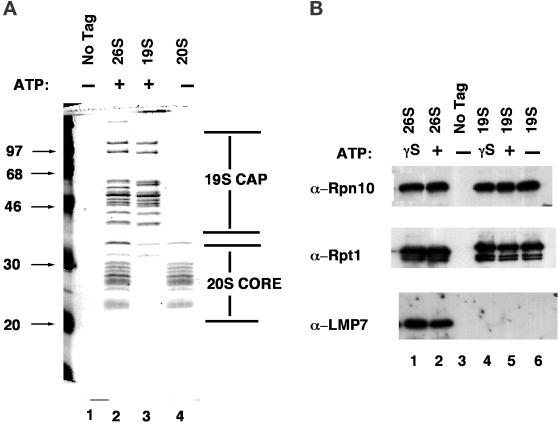
Figure 1.
Affinity purification of 26S proteasomes, plus 19S and 20S subcomplexes. (A) Extracts of yeast strains expressing Pre1FH (RJD 1144, lanes 2 and 4) or Rpt1FH (RJD 1171, lane 3) were prepared and bound to anti-Flag M2 resin as described in MATERIALS AND METHODS. Bound proteins were eluted with 100 μg/ml Flag peptide and analyzed by SDS-PAGE and Coomassie blue staining. The entire purification from the PRE1FH strain was carried out either in the presence (lane 2) or absence (lane 4) of ATP. The purification from the RPT1FH strain was carried out in the presence of ATP (lane 3). Lane 1 depicts a control purification performed with extract from an untagged strain (RJD 487) in the absence of ATP. (B) 26S proteasomes, 19S caps, and mock samples were prepared as described in A from RJD 1144, RJD 1171, and RJD 487, respectively, either in the absence of ATP or in the presence of ATP or ATP-γ-S, as indicated in the figure. Purified samples were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with polyclonal antisera against the mammalian 20S subunit LMP7, which cross-reacts with budding yeast Pre2/Doa3, and the budding yeast 19S subunits Rpn10 and Rpt1.