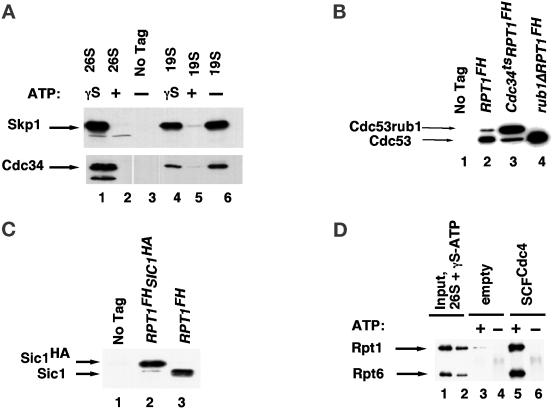
Figure 4.
SCFCdc4 ubiquitin ligase interacts with the proteasome in a nucleotide-modulated manner. (A) 26S proteasomes (from RJD 1144) and 19S caps (from RJD 1171), prepared in the absence of nucleotide or presence of ATP or ATP-γ-S, as indicated in the figure, were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted for the SCF subunit Skp1 and the cognate E2 Cdc34 by using affinity-purified polyclonal antibodies. As a mock control, purification was carried out with extracts from untagged RJD 487 cells (no tag). (B) 19S caps (−ATP) were prepared from untagged (RJD 487, lane 1), RPT1FH (RJD 1171, lane 2), RPT1FHcdc34ts arrested at 37°C for 6 h (RJD 1281, lane 3), and RPT1FHrub1Δ (RJD 1379, lane 4) cells. Aliquots were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with a polyclonal antibody to the SCF subunit Cdc53. Cdc53rub1 refers to Cdc53 conjugated with the ubiquitin-like Rub1 protein. (C) 19S caps (−ATP) were prepared from untagged (RJD 487, lane 1), RPT1FH SIC1HAcdc34-2 (RJD 1294, lane 2), or RPT1FH (RJD 1171, lane 3) cells. Aliquots were resolved by SDS-PAGE and immunoblotted with polyclonal antibody to Sic1. (D) 26S Proteasomes purified on anti-Flag resin in the presence of ATP were washed free of ATP before being eluted with Flag peptide in the presence of 2 mM ATP-γ-S. Tetrameric SCFCdc4 tagged with a polyoma epitope on the Cdc4 subunit was produced in baculovirus cells and retrieved on antipolyoma resin as described in Seol et al. (1999). Eluted 26S proteasomes (10 μg) were mixed with naked (lanes 3 and 4) or ∼0.5 μg of SCF-coated (lanes 5 and 6) antipolyoma beads in the presence or absence of 2 mM ATP, as indicated. After incubation, polyoma beads were washed and evaluated for their content of 26S proteasome by immunoblotting with anti-Rpt1 and anti-Rpt6 sera. Input lanes 1 and 2 contain 14 and 5% respectively, and bound lanes contain 33% of the material from a single binding reaction.