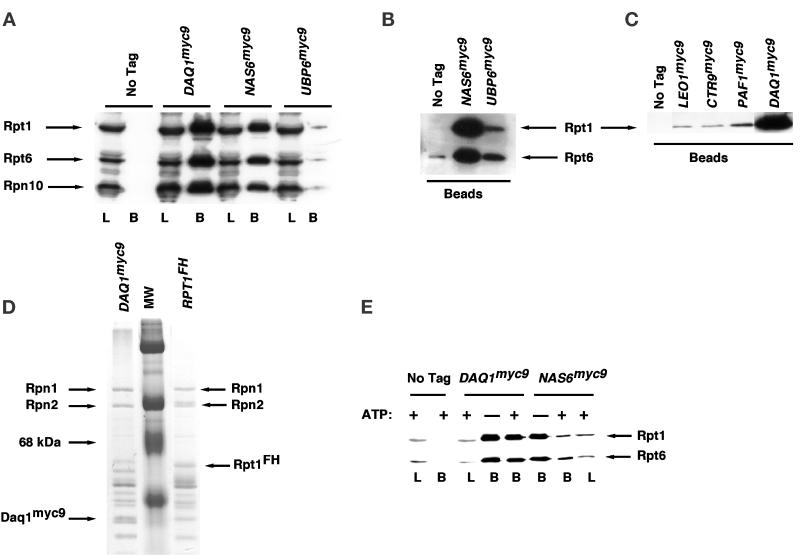
Figure 6.
Coimmunoprecipitation of PIPs with the 19S regulatory particle. (A–C) A subset of the genes listed in Table 3 was modified to encode proteins tagged with the TEV2myc9 epitope, and extracts from the resulting tagged strains were prepared as described in MATERIALS AND METHODS. Tagged antigens were immunoprecipitated with anti-myc antibody covalently coupled to protein A, and immunoprecipitates were evaluated for their content of 19S subunits by SDS-PAGE followed by immunoblotting with polyclonal antibodies to Rpt1, Rpt6, and Rpn10, as indicated. In A, L refers to extract used for immunoprecipitation (3% of input) and B refers to the washed and SDS-eluted immunoprecipitates (Beads, 25% of bound). In B and C, only the washed immunoprecipitates (Beads) were evaluated. The experiment shown in B is similar to that shown in A, except that the blot was allowed to develop longer to reveal the specific coimmunoprecipitation of Rpt1 and Rpt6 with Ubp6myc9. (D) Equivalent amounts of extracts from DAQ1myc9 (RJD 1487) and RPT1FH (RJD 1171) were immunoprecipitated in the absence of ATP by using α-myc and α-flag beads, respectively. Equal aliquots of each immunoprecipitate were visualized by Coomassie blue staining. Rpn1 and Rpn2 are highlighted to indicate that equivalent amounts of bona fide 19S subunits are recovered in Daq1myc9 and Rpt1FH immunoprecipitates. (E) Extracts from DAQ1myc9, NAS6myc9, and cells containing no tagged proteins were immunoprecipitated by using α-myc–coated beads in the presence and absence of ATP, as indicated in the figure. Aliquots were resolved on SDS-gels and immunoblotted with anti-Rpt1/Rpt6 polyclonal antibodies. L refers to 2% of the extract loaded for immunoprecipitation, and B refers to the washed and SDS-eluted immunoprecipitates (Beads, 33% of bound).