Figure 3.
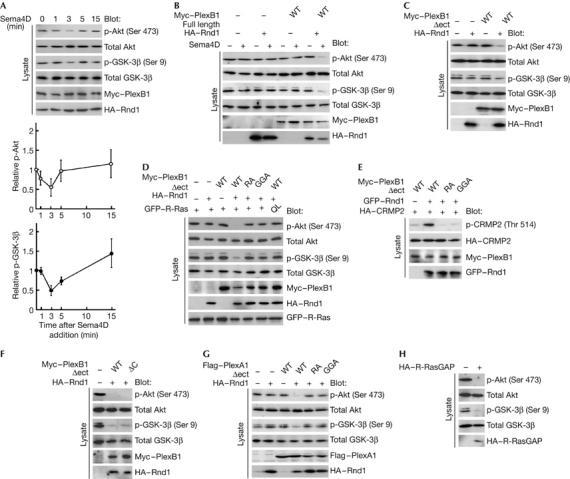
Sema4D dephosphorylates Akt and GSK-3β and phosphorylates CRMP2 through the conserved R-Ras GAP domain within plexin-B1. (A) Time course of Sema4D-induced dephosphorylation of Akt and GSK-3β. COS-7 cells expressing full-length plexin-B1 and Rnd1 were stimulated with Sema4D for the indicated times. The cell lysates at the indicated times were analysed by immunoblot analysis with the phospho-specific antibodies against Akt and GSK-3β. (B) Requirement of Rnd1 in Sema4D-induced dephosphorylation of Akt and GSK-3β. COS-7 cells expressing the indicated plasmids were stimulated with Sema4D for 3 min, and the cell lysates were analysed by immunoblot analysis with phospho-specific antibodies against Akt and GSK-3β. (C–F) Effects of plexin-B1Δect mutants on phosphorylation of Akt, GSK-3β and CRMP2. Lysates from COS-7 cells expressing the indicated plasmids were analysed by immunoblot analysis with phospho-specific antibodies against Akt, GSK-3β and CRMP2. (G) Plexin-A1-mediated dephosphorylation of Akt and GSK-3β. Lysates from COS-7 cells expressing plexin-A1Δect mutants and Rnd1 were analysed by immunoblot analysis with phospho-specific antibodies against Akt and GSK-3β. (H) Dephosphorylation of Akt and GSK-3β by suppression of R-Ras activity. Lysates from COS-7 cells expressing myr-R-RasGAP were analysed by immunoblot analysis with phospho-specific antibodies against Akt and GSK-3β. The results shown are representative of two or three experiments. CRMP2, collapsin response mediator protein 2; GAP, GTPase-activating protein; GFP, green fluorescent protein; GSK-3β, glycogen synthase kinase-3β; HA, haemagglutinin; Sema4D, semaphorin 4D; WT, wild type.
