Figure 6.
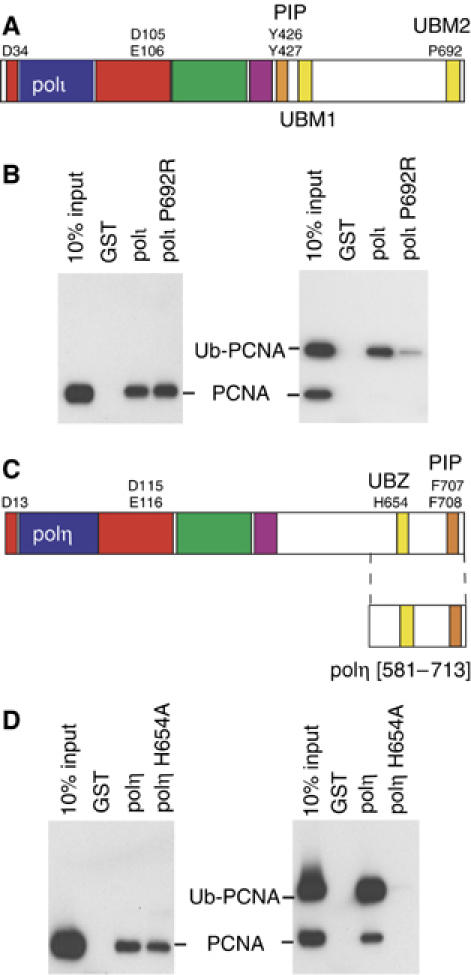
Protein–protein interactions regulating TLS. (A) Schematic representation of the domain/motif structure of polι. The polymerase domain representing conserved finger (blue) palm (red), thumb (green) and little finger (purple) spans the N-terminal portion of each protein. The PCNA binding site (PIP-Box) of each protein is colored orange and the UBMs are in yellow. UBM1 spans residues 496–524 and UBM2 spans amino acids 681–709 (Bienko et al, 2005). Residues identified in the active site of the polymerase, PIP-box or Ub interactions are shown by the single letter code. (B) Full-length wild-type GST-polι or polι-P692R mutant were used in pull-down assays with either PCNA or ubiquitinated-PCNA, separated on 10–20% Tris–glycine–SDS gels, transferred onto PVDF membrane, and PCNA was detected by Western Blot. Left: Both wild-type, and P692R polι GST-fusion proteins are able to bind to PCNA. Right: However, polι-P692R has a reduced ability to pull down Ub-PCNA. (C) Schematic representation of the domain/motif structure of polη (same color scheme as (A)), with amino acids 631–659 comprising the UBZ domain (Bienko et al, 2005). (D) Pull-down assays as described above were carried out with GST fused to the C-terminal 133 amino acids of polη or polη-H654A. Left: GST-polη[581–713] contains the PIP box and both wild-type polη, or the H654A polη mutant can bind PCNA, in accord with data shown in Figure 4B. Right: However, while the wild-type polη protein is able to pull-down Ub-PCNA, the H654A polη-Ub mutant is unable to bind ubiquitinated PCNA.
