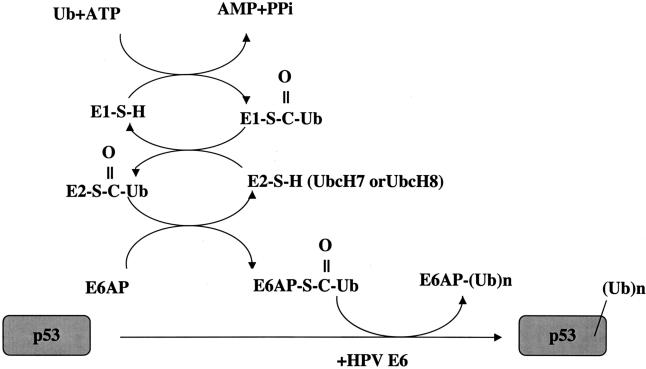
Fig. 4.
HPV E6-mediated ubiquitylation of p53. E6 binds the cellular protein E6AP to functions as an E3 (ubiquitin protein ligase in the ubiquitylation of p53 (23). The ubiquitylation of a protein involves three cellular activities: E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme), and E3 (ubiquitin protein ligase). Ubiquitin is activated in an ATP dependent manner and bound to E1 through a high energy thiolester bond. It is then transferred to the E2 ubiquitin conjugating enzyme, again through a thiolester linkage. Ubiquitin can then be transferred to a cysteine within the HECT domain of E6AP, again as a thiolester linkage (46) through the direct binding of E6AP with UbcH7 or UbcH8 (47). In conjunction with HPV-16 E6, E6AP then recognizes p53 and catalyses the formation of an isopeptide bond between the carboxy-terminal glycine of ubiquitin and a lysine side chain of p53. Noteworthy is that the turnover of E6AP itself is increased in the presence of E6, presumably a result of its increased enzymatic activity mediated by E6.