Figure 5.
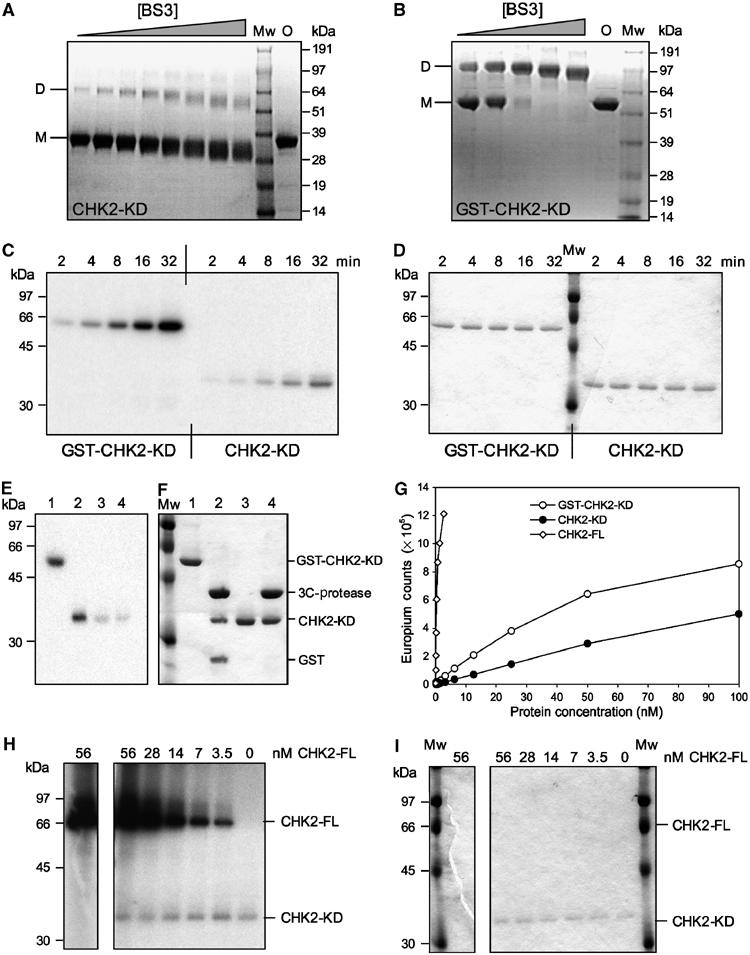
Dimerisation, Auto- and substrate-phosphorylation of CHK2-KD. (A) SDS–PAGE analysis of CHK2-KD crosslinking. (B) SDS–PAGE analysis of GST-CHK2-KD crosslinking. In each case, a fixed concentration of protein was incubated with increasing concentrations of the homo-bifunctional crosslinker BS3. Mw=molecular mass marker, O=protein not exposed to crosslinking agent, D=dimeric species, M=monomeric species. (C) Autophosphorylation assay. Fixed concentrations of GST-CHK2-KD or CHK2-KD were incubated with γ-32P-labelled ATP for increasing periods of time (as indicated). Samples were analysed by SDS–PAGE, visualised by phosphorimager and autoradiography. (D) Coomassie-stained SDS–PAGE gel of (C). (E) Autophosphorylation specifically occurs within the kinase domain of Chk2. Autophosphorylated samples of GST-CHK2-KD (Lane 1) and CHK2-KD (3) were incubated with rhinovirus 3C-protease (Lanes 2 and 4, respectively) then analysed by SDS–PAGE. Incorporation of γ-32P-labelled ATP was visualised by autoradiography. (F) Coomassie-stained SDS–PAGE gel of (E). (G) DELFIA assay. Samples of CHK2-FL, GST-CHK2-KD and CHK2-KD at varying concentrations were incubated, in the presence of ATP, with a substrate peptide (corresponding to the sequence flanking the Ser216 phosphorylation site of CDC25C). Phosphorylation was detected by incubation with a polyclonal anti-CDC25C(Ser216) antibody, followed by a Europium-labelled secondary antibody. A fluorescent plate reader was then used to quantitate the level of peptide phosphorylation (as Europium counts). (H) Trans-phosphorylation assay. Increasing amounts of CHK2-FL were incubated with a fixed concentration of CHK2-KD (as indicated) in the presence of γ-32P-labelled ATP. Samples were analysed by SDS–PAGE, then visualised by phosphorimager and autoradiography. (I) Coomassie-stained SDS–PAGE gel of (H).
