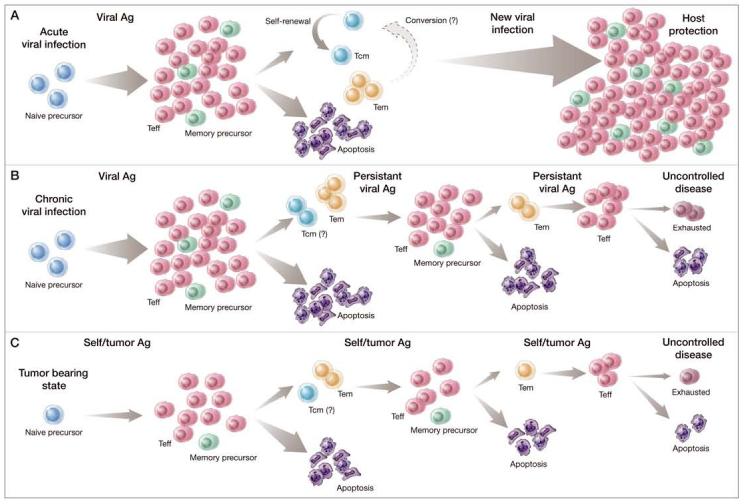
Figure 1.
Comparison of normal and corrupted CD8+ T-cell memory formation. CD8+ T-cell memory formation in the settings of an acute viral infection (A), chronic viral infection (B), and the tumor-bearing state (C) begins when a population of naive, antigen (Ag)-specific CD8+ T cells (blue cells) are stimulated to divide by exposure to cognate Ag. A productive encounter with cognate Ag induces a multi-log clonal expansion of this naïve precursor pool. As these cells divide, they acquire the phenotypic and functional attributes of terminally differentiated effector T cells (TEFF) (red cells) cells that allow these cells to clear the stimulating Ag. At the conclusion of the 1o response, the majority (∼90%) of responding CD8+ T cells die by apoptosis; a limited subset of TEFF cells (∼10%, green cells) advance to form a stable pool of memory CD8+ T cells. The memory pool is heterogeneous and can be divided into self-renewing central memory T cells (TCM) (aquamarine cells) and effector memory T cells (TEM) cells (yellow cells). Whether TEM convert to TCM with time is the current subject of controversy. Under normal conditions, both pools of memory T cells contribute to host protection from future pathogen challenge. In the settings of a chronic viral infection or the tumor-bearing state, the normal pattern of CD8 + memory T-cell production can be altered. With time, memory cells are driven to a highly differentiated or ‘exhausted’ phenotypic and functional state (purple cells), culminating in some cases in the deletion of responding cells. Ultimately, the memory CD8+ T cells generated under these conditions are unable to efficiently clear the challenging Ag, resulting in uncontrolled disease.