Abstract
The synthesis of 14S pentamers and 70S empty capsids of hepatitis A virus (HAV) has been accomplished by expressing the viral genome for periods of time longer than 4 h in Escherichia coli. HAV pentamers (14S) self-assembled into capsids (70S) in vitro. The antibodies induced by these structures recognized and neutralized HAV.
The immunodominant neutralization antigenic site of the hepatitis A virus (HAV) is composed of closely related epitopes: some of them are detected on 14S pentameric subunits, while others are formed by structural changes during assembly of 14S structures into 70S and intact particles (9). Assembly of capsid proteins into subviral or virion structures might then be necessary for the generation of efficient HAV-neutralizing epitopes.
The expression of the complete open reading frame of the HAV genome in Escherichia coli gives mainly an insoluble fraction, containing the unprocessed P1 polyprotein (1), and a soluble fraction constituted by the processed structural proteins (5). To evaluate the usefulness of E. coli as an eventual expression system for the production of HAV structured antigens, antigenic and structural analysis of the viral maturation process was performed at different times postexpression (5).
E. coli strain JM109 harboring the pTHAVF plasmid, which expresses the complete HAV open reading frame, was grown on M9 medium supplemented with 0.4% glucose and 50 μg of ampicillin/ml. When the bacterial growth was at the beginning of the exponential phase (optical density at 600 nm [OD600], around 0.4), expression of the genome under the Tac promoter control was induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). At different times postinduction, bacterial cells from 50 ml of culture were resuspended in 500 μl of TNE buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, pH 7.4) and lysozyme treated (1 mg/ml) for 1 h. After three freeze-thawing cycles at −70°C, MgCl2 was added to achieve a final concentration of 10 mM and cell extracts were incubated with DNase I at a 10 μg/ml concentration at 4°C for 2 h. Two different fractions were recovered after centrifugation of the bacterial lysates at 11,000 × g for 10 min: an insoluble protein fraction, in the form of inclusion bodies (i.b.) corresponding to the pellet, and a soluble protein fraction corresponding to the supernatant.
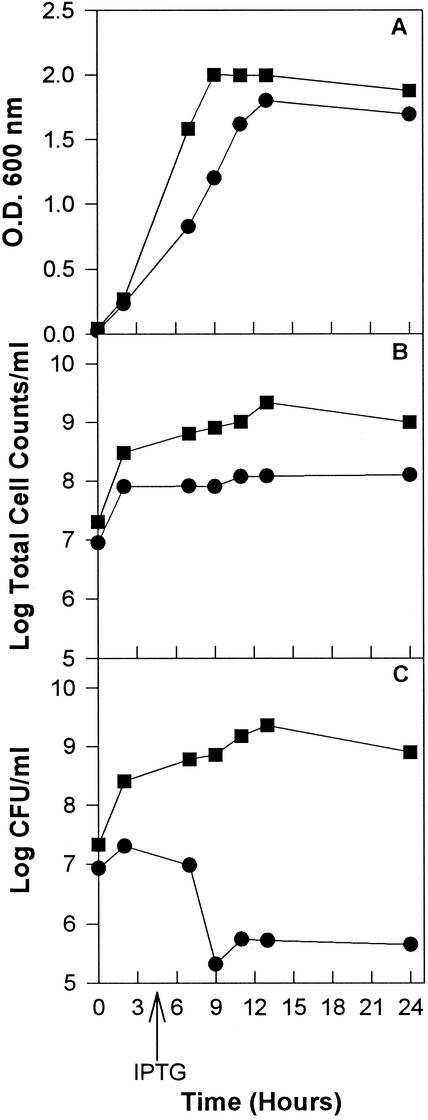
The detection of HAV antigenic material in the soluble supernatant was performed by a direct enzyme-linked immunosorbent assay (ELISA), using a polyclonal murine ascitic antibody against intact HAV particles (anti-HAVs) (4). Bacterial extracts from cultures harboring the pBTac-2 parental plasmid were used as negative controls. The soluble antigenicity increased over time (Table 1). This increment might be due either to an increase in the de novo synthesis of soluble antigenic material or to an antigenic maturation of the previously synthesized material. The bacterial growth was monitored by measuring the OD600, by counting the number of viable cells (in CFU per milliliter) on LB agar plates, and by counting the number of total cells (in cells per milliliter) after staining with the fluorochrome DAPI (4′,6′diamidino-2-phenylindole), and it was observed that the viability of the pTHAVF-bearing recombinant strain declined after 4 h of induction (Fig. 1). Therefore, it was difficult to discern a real increase in the recombinant protein concentration. It has been recently postulated that i.b. are not merely irreversible accumulations of misfolded recombinant proteins but are reversible protein aggregations that release properly folded native proteins to the soluble cell compartment when protein synthesis is arrested (2, 3). Consequently, in experiments like ours, this phenomenon might lead to an increase in the concentration of those conformations able to produce subviral and viral structures.
TABLE 1.
Detection of HAV antigens in cell-free extracts of E. coli cells harboring pBTac-2 or pTHAVF plasmids at different times after the induction of protein synthesis
| Plasmid | OD492 (± SD) by ELISA at the indicated h postinduction
|
||
|---|---|---|---|
| 4 | 8 | 20 | |
| pBTac | 0.215 ± 0.0007a | 0.227 ± 0.02 | 0.185 ± 0.03 |
| pTHAVF | 0.285 ± 0.07 | 0.354 ± 0.05 | 0.498 ± 0.003 |
The threshold of positivity was established by adding 3 standard deviations to the mean of the negative pBTac control.
FIG. 1.
Growth of E. coli cells harboring either the parental pBTac2 plasmid (square) or the recombinant pTHAVF plasmid (circle). Growth monitoring was performed by measuring the OD600 (A), by counting the total number of cells by DAPI staining (B), and by counting the viable cells (C).
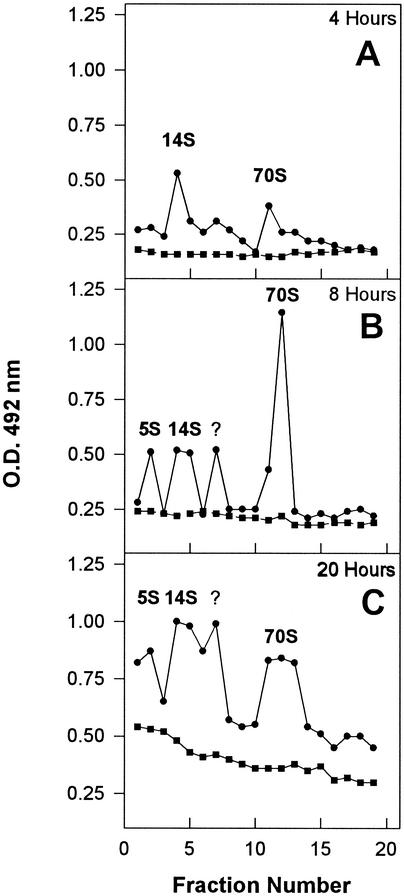
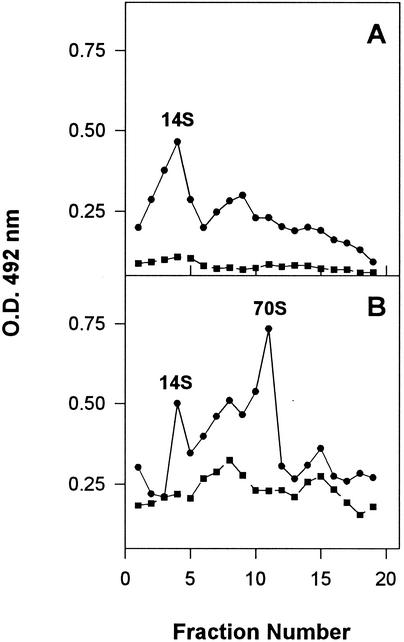
To confirm the nature and the amount of the antigenic material, sucrose gradient analysis of the i.b.-free bacterial extracts was performed. A total of 500 μl of the soluble fraction extracted after the expression of pTHAVF was submitted to three cycles of sonication at 70 W for 30 s and was subsequently layered onto a 5 to 45% sucrose gradient in TNMg buffer (20 mM Tris-HCl, 10 mM NaCl, 50 mM MgCl2, pH 6.7) and spun at 205,000 × g for 165 min. Fractions (500 μl) were collected, and the presence of HAV antigenic material and refraction indices was determined for each fraction. HAV-related antigens were detected by a sandwich ELISA, consisting of HAV capture by human convalescent-phase serum 2 (HCS-2) (4), followed by detection with the 14S epitope-specific monoclonal antibody K2-4F2 (Commonwealth Serum Laboratories, Victoria, Australia). Sucrose gradient fractions of pBTac-2 bacterial extracts were used as negative controls. After 4 h of induction, two antigenic peaks with sedimentation coefficients of 14S and 70S were detected (Fig. 2A). After an 8-h induction, besides a significant increase in the 70S peak, two new antigenic peaks emerged, one around 5S and another between 14S and 70S (Fig. 2B). After 20 h of induction, the antigenic concentration greatly increased for all the peaks, taking into consideration that the 70S peak became a plateau (Fig. 2C).
FIG. 2.
Soluble HAV antigens from cells harboring the parental pBTac2 (square) or the recombinant pTHAVF (circle) plasmids at different times postinduction. The different viral structures were separated by sucrose gradient centrifugation and detected by a sandwich ELISA consisting of capture of HAV antigens through use of the convalescent-phase serum HCS-2 and detection through use of the monoclonal antibody K24F2.
To assay the maturation capability of some of the subviral structures, pooled sucrose fractions, corresponding to the 14S pentamers or 70S capsids purified from around 109 bacterial cells, were submitted to dialysis for sucrose removal, concentrated to a final volume of 500 μl by methanol precipitation, and layered onto a new gradient. The pooled 70S fractions gave the same 70S antigenic peak (data not shown). However, pooled 14S fractions resulted in the generation of both 14S and (mainly) 70S peaks (Fig. 3), demonstrating that self-assembly had occurred in vitro. That 14S structures self-assemble in vitro into 70S empty capsids has been described for poliovirus (6) and for recombinant HAV structures expressed in the vaccinia virus system (9). The other two peaks were not tested because of their unknown nature. The 5S peak might potentially correspond to protomers or merely to denatured HAV proteins.
FIG. 3.
Soluble HAV antigens from cells harboring the parental pBTac2 (square) or the recombinant pTHAVF (circle) plasmids at 4 h postinfection (A). Fractions corresponding to the 14S peak were pooled and submitted to a second sucrose gradient centrifugation (B). HAV detection was performed as described for Fig. 2.
Since the 14S pentamers self-assembled to 70S capsids, a total i.b.-free crude extract, containing in theory both 14S and (mainly) 70S structures, was administered to mice to test their immunogenic potential. The number of 70S capsids contained in these suspensions was estimated by immunoprecipitation-Western blotting (5), the threshold of sensitivity of this technique being 5 × 106 HAV particles. Since the direct bacterial crude supernatant (500 μl) used for the inoculation of mice was recorded as positive under this latter method and its 1/10 dilution was recorded as negative, we assumed that the titer in the supernatant was around 1 × 107 particles/ml. An i.b. preparation with a protein concentration of 100 mg/ml was sonicated in the presence of 0.5% sodium lauryl sulfate and was then administered to mice. Female Swiss mice (6 weeks old) were used to produce ascitic antibodies, after immunization with the different recombinant products employing Freund's complete adjuvant (4). A total of 200 μg of protein per dose in the case of i.b., and around 2 × 105 recombinant particles per dose in the case of the supernatant product, was administered. Ascites generated by inoculation of phosphate-buffered saline were used as negative controls, while ascites generated by inoculation of 2 × 107 HAV intact particles per dose were used as positive controls. HAV recognition by the ascitic fluids was evaluated by a sandwich ELISA in which HAV was captured by the HCS-2 convalescent-phase serum and detected by the different ascitic fluids tested (4). After a 3-hour incubation at 37°C, ascitic fluids were also assayed for the capacity to neutralize the infectivity of the cytopathogenic HM-175 strain of HAV, as described elsewhere (4). The comparative immunogenicity of the different recombinant products versus that of the virus is shown in Table 2. The response generated with the recombinant structures may be considered quite efficient, keeping in mind that the dose inoculated was 100-fold lower than that of the intact virus. HAV intact particles induced a homogeneous immunogenic response, with high antibody titers both for the virus recognition (1/100,000) and for a 90% neutralization (1/5,000). In the case of the recombinant 14S and 70S structures, the response was of lower magnitude and more heterogeneous, since only 60% of the inoculated mice responded. The titer for the virus recognition ranged from 1/10,000 to 1/50,000. The maximum percentages of neutralization observed were 73, 75, and 90% at dilutions of 1/500, 1/2,000, and 1/2,000, respectively. All of these percentages of neutralization exceeded the 60% cutoff established by others (7). However, higher percentages of neutralization were never achieved, even after concentration of the antibody suspensions, which suggests that some epitopes of the intact HAV virion were not present in the 70S recombinant capsids. In comparison, the 14S-bearing i.b. did not elicit a good immunogenic response, the maximum neutralization being 66% at a very high concentration of antibodies. However, this result might have been due to the inaccessibility of the 14S epitope in the i.b. (1).
TABLE 2.
Comparative immunogenicity of recombinant products versus that of the HM-175 strain of HAV
| Immunogen | Total antigen (ng) | Anti-HAV titera (no. of mice positive for HAV recognition/total no. of mice) | Neutralization titerb (% HAV neutralization) |
|---|---|---|---|
| 14S i.b. | 200,000 | NTc | 1/4 (66) |
| 14S + 70S VLPd | 0.01 | 1/10,000 | 1/500 (73) |
| 1/50,000 | 1/2,000 (75) | ||
| 1/50,000 (3/5) | 1/2,000 (90) | ||
| 70S + 150S HAV | 1 | 1/100,000 (6/6) | 1/5,000 (90) |
Values represent the highest ascitic fluid dilutions positive for HAV recognition.
Fractional values represent ascitic fluid dilutions.
NT, not tested.
VLP, virus-like particles.
The synthesis of 70S immunogenic HAV recombinant capsids is feasible in E. coli after long expression times. The yield of virus-like particles is low, however, possibly due to the highly codon usage-dependent capsid folding of HAV (8) and the nonconcordant codon usages of the virus and the expression host, among other factors. The efficiency of production might be increased if methods for the release of refolded i.b-associated proteins become available.
Acknowledgments
This study was supported in part by grants QLRT-1999-0634 from the European Union, BIO95-0061 and BIO99-0455 from the CICYT, Ministry of Science and Technology, Spain, and 2001/SGR/00098 from the Generalitat de Catalunya and by the Centre de Biotecnologia de Catalunya (CeRBA), Generatitat de Catalunya.
REFERENCES
- 1.Bosch, A., K. J. Guo, J. F. González-Dankaart, X. Arnijas, S. Guix, G. Sánchez, E. Ribes, and R. M. Pintó. 1997. Recombinant hepatitis A virus polyprotein expressed in E. coli assembles in subviral structures, p. 27-31. In M. Rizzeto, R. H. Purcell, J. L. Gerin, and G. Verme (ed.), Viral hepatitis and liver disease. Edizioni Minerva Medica, Turin, Italy.
- 2.Carrió, M. M., R. Cubarsi, and A. Villaverde. 2000. Fine architecture of bacterial inclusion bodies. FEBS Lett. 471:7-11. [DOI] [PubMed] [Google Scholar]
- 3.Carrió, M. M., and A. Villaverde. 2001. Protein aggregation as bacterial inclusion bodies is reversible. FEBS Lett. 489:29-33. [DOI] [PubMed] [Google Scholar]
- 4.Pintó, R. M., J. F. González-Dankaart, G. Sánchez, S. Guix, M. J. Gómara, M. García, I. Haro, and A. Bosch. 1998. Enhancement of the immunogenicity of a synthetic peptide bearing a VP3 epitope of hepatitis A virus. FEBS Lett. 438:106-110. [DOI] [PubMed] [Google Scholar]
- 5.Pintó, R. M., S. Guix, J. F. González-Dankaart, S. Caballero, G. Sánchez, K. J. Guo, E. Ribes, and A. Bosch. 2002. Hepatitis A virus polyprotein processing by Escherichia coli proteases. J. Gen. Virol. 83:359-368. [DOI] [PubMed] [Google Scholar]
- 6.Rombaut, B., A. Foriers, and A. Boeye. 1991. In vitro assembly of poliovirus 14S subunits: identification of the assembly promoting activity of infected cell extracts. Virology 180:781-787. [DOI] [PubMed] [Google Scholar]
- 7.Rosen, E., J. T. Stapleton, and J. McLiden. 1993. Synthesis of immunogenic hepatitis A virus particles by recombinant baculoviruses. Vaccine 11:706-712. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez, G., A. Bosch, and R. M. Pintó. Genome variability and capsid structural constraints of hepatitis A virus. J. Virol. 77:452-459. [DOI] [PMC free article] [PubMed]
- 9.Stapleton, J. T., V. Raina, P. L. Winokur, K. Walters, D. Klinzman, E. Rosen, and J. H. McLinden. 1993. Antigenic and immunogenic properties of recombinant hepatitis A virus 14S and 70S subviral particles. J. Virol. 67:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]