Abstract
The PhoP/PhoQ two-component regulatory system of Salmonella enterica serovar Typhimurium plays an essential role in controlling virulence by mediating the adaptation to Mg2+ depletion. The pho-24 allele of phoQ harbors a single amino acid substitution (T48I) in the periplasmic domain of the PhoQ histidine kinase sensor. This mutation has been shown to increase net phosphorylation of the PhoP response regulator. We analyzed the effect on signaling by PhoP/PhoQ of various amino acid substitutions at this position (PhoQ-T48X [X = A, S, V, I, or L]). Mutations T48V, T48I, and T48L were found to affect signaling by PhoP/PhoQ both in vivo and in vitro. Mutations PhoQ-T48V and PhoQ-T48I increased both the expression of the mgtA::lacZ transcriptional fusion and the net phosphorylation of PhoP, conferring to cells a PhoP constitutively active phenotype. In contrast, mutation PhoQ-T48L barely responded to changes in the concentration of external Mg2+, in vivo and in vitro, conferring to cells a PhoP constitutively inactive phenotype. By analyzing in vitro the individual catalytic activities of the PhoQ-T48X sensors, we found that the PhoP constitutively active phenotype observed for the PhoQ-T48V and PhoQ-T48I proteins is solely due to decreased phosphatase activity. In contrast, the PhoP constitutively inactive phenotype observed for the PhoQ-T48L mutant resulted from both decreased autokinase activity and increased phosphatase activity. Our data are consistent with a model in which the residue at position 48 of PhoQ contributes to a conformational switch between kinase- and phosphatase-dominant states.
Two-component regulatory systems allow bacteria to adapt to a variety of environmental changes by modulating the expression of specific genes. The Salmonella enterica serovar Typhimurium PhoP/PhoQ two-component system plays an essential role in coordinating the expression of various virulence factors (9, 23). By modulating the expression of more than forty different genes, it controls adaptation to Mg2+-limiting environments, protein secretion by the type III secretion system located on Salmonella pathogenicity island 1, entry into epithelial cells, survival within macrophages, lipopolysaccharide modifications, and resistance to antimicrobial peptides (reviewed in references 8 and 12).
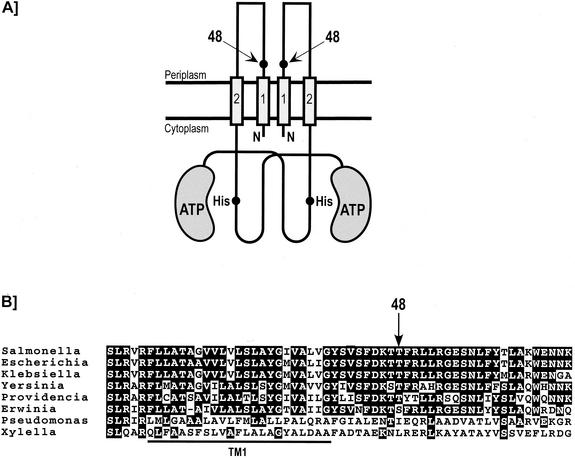
The PhoQ protein is a transmembrane histidine kinase sensor that is present in the inner membrane as a homodimer (Fig. 1). Each subunit consists of an N-terminal periplasmic ligand-recognition domain (residues 40 to 194) flanked by two transmembrane segments (TM1 and TM2) and a C-terminal cytoplasmic signaling domain (residues 215 to 487). The PhoQ protein has been shown to sense some external divalent cations such as Mg2+, Ca2+, and Mn2+ (10). It has been proposed that Mg2+ is the physiological signal controlling the PhoP/PhoQ system (11). Limiting concentrations of Mg2+ in the extracellular environment activate the cytoplasmic phosphorylation cascade by promoting the ATP-dependent autophosphorylation of PhoQ on an invariant His residue (His 277) (3, 26). Subsequently, the phosphoryl group is transferred from PhoQ to a highly conserved Asp residue (Asp 55) of the response regulator PhoP. DNA binding of phospho-PhoP promotes both transcription of PhoP-activated genes and repression of PhoP-repressed genes. In contrast, high concentrations of external Mg2+ stimulate the PhoQ phosphatase activity that dephosphorylates phospho-PhoP (3, 26). In turn, this represses transcription of PhoP-activated genes and derepresses PhoP-repressed genes. Thus, environmental Mg2+ controls the intracellular level of phospho-PhoP by coordinately regulating PhoQ autokinase and phosphatase activities (26).
FIG. 1.
Location of position 48 in the periplasmic domain of PhoQ. (A) Schematic representation of the PhoQ sensor kinase. The location of position 48 is shown by arrows. The cytoplasmic domain representation is based on the nuclear magnetic resonance structures of the E. coli EnvZ sensor kinase (7). It consists of a catalytic ATP-binding domain and a dimerization domain that contains the invariant histidine residue. (B) Partial alignment of the amino acid sequences of the S. enterica serovar Typhimurium PhoQ protein to homologues in E. coli, Klebsiella pneumoniae, Yersinia pestis, Providencia stuartii, Erwinia carotovora, P. aeruginosa, and X. fastidiosa. The arrow indicates position 48 (S. enterica serovar Typhimurium numbering). The bold line under the sequences indicates the N-terminal transmembrane segment (TM1), which has been predicted on the basis of a hydrophobicity plot.
The pho-24 allele of phoQ that was isolated after diethyl sulfate mutagenesis of S. enterica serovar Typhimurium strain LT2 resulted in constitutive expression of phoN, a PhoP-activated gene that encodes a periplasmic acid phosphatase (19). Like Salmonella phoP- or phoQ-defective strains, those strains harboring the pho-24 allele are attenuated for virulence in mice (13, 23, 24). The pho-24 allele confers a PhoP-constitutive phenotype (PhoPc) to cells that is characterized by the constitutive expression and repression of PhoP-activated genes and PhoP-repressed genes, respectively (24). This altered pattern of gene expression results in pleiotropic effects that include decreased invasion of epithelial cells (2, 27) and defects in stimulating macropinocytosis and spacious phagosome formation in macrophages (1). It has subsequently been shown that a change of Thr to Ile at position 48 of the PhoQ protein was responsible for the phenotypes associated with the pho-24 allele (10, 14). Salmonella strains harboring the pho-24 allele of phoQ have been used in various studies to establish the involvement of the PhoP/PhoQ system in lipid A modifications (15), in resistance against host antimicrobial peptides (16, 25), and in resistance to bile (29). In addition, these strains have been used as carriers for heterologous antigens in live oral vaccines (17). Although the pho-24 mutation has been shown to increase net phosphorylation of PhoP (14), the mechanism by which this mutation affects signaling by the PhoP/PhoQ system remains unknown.
Our study assesses the molecular mechanism by which a mutation at position 48 in the PhoQ periplasmic domain affects catalytic activities in the cytoplasmic signaling domain. We generated mutants of the phoQ gene with amino acid substitutions at position 48 and analyzed the catalytic activities of the encoded proteins both in vivo and in vitro.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli strain XL-1 Blue (Stratagene) was used for all DNA manipulations. E. coli strain BL21(DE3)/pLysE (Novagen) was used for protein expression. E. coli strain MG1607 [F− Δ(argF-lac)U169 araD139 rpsL150 ptsF25 fibB5301 rbsR deoC relA1 mgtA::λplacMu55 phoQ608::Tn10dCam], which was kindly provided by R. Utsumi, Kinki University, Japan (18), was used for β-galactosidase analyses. Bacterial cultures were grown at 37°C with aeration in Luria-Bertani (LB) broth containing the appropriate antibiotics (100 μg of ampicillin per ml and 30 μg of chloramphenicol per ml).
Plasmids and DNA manipulations.
Plasmid pET-Q, containing the S. enterica serovar Typhimurium phoQ gene under the control of the T7 promoter (26), was used to generate the series of mutants at position 48 (pET-Q-T48X). Site-directed mutagenesis was performed according to the method of Deng and Nickoloff (6). The native Thr residue at position 48 was replaced by a Ser residue by using primer Q-T48S (5′-AGTTTTGATAAAACCAGCTTTCGTTTGC-3′), by an Ala residue by using primer Q-T48A (5′-GTTTTGATAAAACCGCCTTTCGTTTGCTG-3′), by an Ile residue by using primer Q-T48I (5′-AGTTTTGATAAAACCATCTTTCGTTTGC-3′), by a Val residue by using primer Q-T48V (5′-GTTTTGATAAAACCGTCTTTCGTTTGCTG-3′), and by a Leu residue by using primer Q-T48L (5′-GTTTTGATAAAACCCTCTTTCGTTTGCTG-3′). Plasmid pET-PHis encoded the S. enterica serovar Typhimurium PhoP protein fused to a C-terminal His tag (PhoPHis) (26).
To carry out β-galactosidase analyses, we constructed the plasmid pPRO-QHis, in which the S. enterica serovar Typhimurium phoQ gene fused to a C-terminal His tag is overexpressed under the control of the tac promoter. This medium-copy-number plasmid harboring the p15A origin of replication was derived from pPROLar.A122 (Clontech) by inserting the lacIq coding sequence and replacing the kanamycin resistance gene with the ampR gene. The pPRO-QHis-T48X series of plasmids was constructed by subcloning the various 732-bp KpnI-MluI fragments from the pET-Q-T48X plasmids into pPRO-QHis that had been digested with the same enzymes.
β-Galactosidase assays.
E. coli MG1607 cells transformed with the pPRO-QHis-T48X series of plasmids were grown overnight at 37°C in LB broth supplemented with 0.1% glucose. Cultures were diluted 1:100 in LB broth supplemented with 0.1% glucose in the presence or absence of 10 mM MgCl2. Cultures were grown to an optical density at 600 nm (OD600) of ∼0.8 before induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h. Cell lysates were prepared using reporter lysis buffer (Promega). β-Galactosidase activity was measured, using ONPG (o-nitrophenyl-β-d-galactopyranoside) as a substrate, as previously described (22). Expression levels of the PhoQHis-T48X mutants were monitored by Western blotting. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. His-tagged proteins were detected by using His•Tag monoclonal antibody (Novagen) and His•Tag AP LumiBlot reagents (Novagen) as recommended by the manufacturer.
Preparation of membranes enriched in the various PhoQ-T48X proteins.
E. coli BL21(DE3)/pLysE cells, transformed with the pET-Q-T48X series of plasmids, were grown at 37°C in LB broth supplemented with appropriate antibiotics. Transcription of the phoQ gene was induced at an OD of ∼0.8 by adding 0.5 mM IPTG. After 4 h of induction, cells were harvested by centrifugation and membranes were prepared as described previously (20). Using the standard procedure of the Bio-Rad protein assay in the presence of 0.1% SDS, the total amount of protein in membranes was measured by the Bradford method. Expression of the various PhoQ-T48X proteins was visualized on an SDS-12.5% PAGE gel. After the Coomassie blue-stained gels were scanned, the percentage corresponding to the overproduced PhoQ-T48X proteins in membranes was estimated.
Expression and purification of PhoPHis.
E. coli BL21(DE3)/pLysE cells transformed with the pET-PHis plasmid were grown at 22°C in LB broth supplemented with appropriate antibiotics. Cultures were induced overnight with 1 mM IPTG at an OD of ∼0.8, and cells were harvested by centrifugation, resuspended in loading buffer consisting of 20 mM Tris-HCl (pH 7.9), 500 mM NaCl, 50 mM imidazole, and 1 mM phenylmethylsulfonyl fluoride, and sonicated at 4°C. Cell debris was removed by centrifugation, and the supernatant was loaded onto a 1-ml HiTrap chelating column (Amersham Biosciences). The column was extensively washed with loading buffer and eluted using a 0 to 600 mM imidazole gradient in loading buffer. Fractions containing PhoPHis were pooled and dialyzed extensively against 20 mM Tris-HCl (pH 7.5)-50 mM NaCl-10% glycerol. The concentration of purified PhoPHis was measured using the Bio-Rad protein assay.
In vitro reconstitution of the PhoP/PhoQ signaling cascade.
The net phosphorylation of PhoP was measured by incubating membranes containing the PhoQ-T48X proteins (approximately 1.5 μM PhoQ-T48X) with a sixfold molar excess of PhoPHis in a 15-μl volume of phosphorylation buffer (50 mM Tris-HCl [pH 7.5], 200 mM KCl, 0.1 mM EDTA, 5% glycerol) supplemented with 5 mM MgCl2. Reactions were initiated by the addition of 0.1 mM [γ-32P]ATP (10 Ci/mmol), incubated for 20 min at 22°C to reach an apparent steady state, and stopped by addition of 4× Laemmli SDS sample buffer (250 mM Tris-HCl [pH 6.8], 8% SDS, 40% glycerol, 0.02% bromophenol blue, 4% β-mercaptoethanol). Reaction products were heated at 37°C for 3 min and applied to SDS-10% PAGE gels. Gels were dried and exposed to a PhosphorImager (Molecular Dynamics). Using Image Quant software (Molecular Dynamics), phosphorylated proteins were quantified by image analysis.
In vitro autophosphorylation assays.
Membranes containing the PhoQ-T48X proteins (approximately 1.5 μM PhoQ-T48X) were autophosphorylated with 0.1 mM [γ-32P]ATP (10 Ci/mmol) in a 15-μl volume of phosphorylation buffer supplemented with 0.1 mM MgCl2 (26). The phosphorylation reactions were continued at 22°C for various times before being stopped by addition of 4× Laemmli SDS sample buffer. Reaction products were analyzed by SDS-PAGE as described above.
In vitro phosphatase assays.
Purified PhoPHis was phosphorylated with membranes containing the overexpressed PhoQ-T48V protein in a 75-μl volume of phosphorylation buffer supplemented with 5 mM MgCl2 and 0.1 mM [γ-32P]ATP (10 Ci/mmol). After 20 min of incubation at 22°C, the reaction mixture was centrifuged at 16,000 × g for 10 min at 4°C to pellet membranes. The supernatant was loaded on a Micro Bio-Spin chromatography column (Bio-Rad) to remove unincorporated ATP, generated ADP, and MgCl2. The flowthrough containing phospho-PhoPHis was immediately used for the phosphatase assay. Phospho-PhoPHis (15 μM) was mixed with membranes containing the PhoQ-T48X proteins (approximately 1.5 μM PhoQ-T48X) in a 15-μl volume of phosphorylation buffer supplemented with 10 mM MgCl2. At various time points, reactions were stopped by the addition of 4× Laemmli SDS sample buffer. Phosphorylated products were analyzed as described above.
RESULTS
Isolation of the PhoQ-T48X mutations.
The residue at position 48 is located at the N terminus of the periplasmic domain of PhoQ, in close proximity to the first transmembrane segment (TM1) (Fig. 1A). Secondary structure predictions of the PhoQ periplasmic domain suggested that the Thr at position 48 is part of an α-helix that is probably continuous with the postulated α-helical TM1 (21). Although the Thr residue at position 48 is conserved in many PhoQ homologues, other amino acid residues (Ser, Ile, or Leu) are present at this position in the most distantly related PhoQ homologues (Fig. 1B). To understand the molecular mechanism by which the mutation T48I (the pho-24 allele of phoQ) affects the PhoQ catalytic activities, we used site-directed mutagenesis (6) to replace the native Thr residue with Ala, Ser, Val, Ile, and Leu residues. The rationale in mutating the Thr at position 48 of PhoQ is based on the polarity, size, and hydrophobicity of the side chains.
Effect of the PhoQ-T48X mutations on the expression of a PhoP-activated gene.
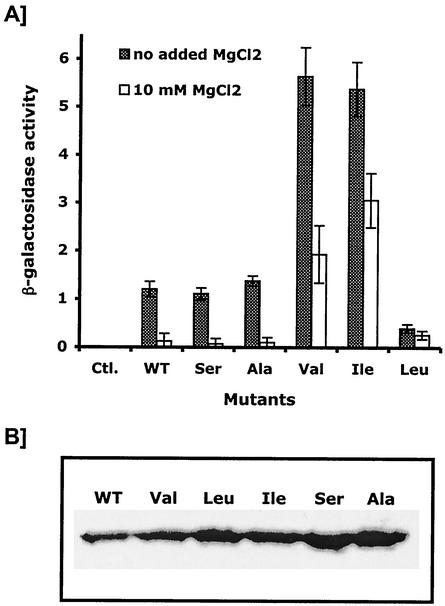
To examine the effects of the PhoQ-T48X mutations on the entire PhoP/PhoQ signaling pathway in vivo, we measured the expression of mgtA (a PhoP-activated gene that encodes a Mg2+ transporter) by measuring the β-galactosidase activity of the mgtA::lacZ transcriptional fusion (18). E. coli MG1607 cells (a phoQ-defective strain) (18) were transformed with the various pPRO-QHis-T48X plasmids encoding the S. enterica serovar Typhimurium PhoQ mutated proteins. To quantify the expression of the PhoQ-T48X proteins by Western blotting, we used plasmids encoding the PhoQ protein fused to a C-terminal tag of 6 histidine residues (PhoQHis-T48X). The catalytic activities of the PhoQHis protein were essentially similar to those of the untagged PhoQ protein, as judged by both in vivo and in vitro assays (data not shown).
As found in previous studies (10, 28), β-galactosidase activity of cells producing wild-type PhoQHis was decreased by about 10-fold when LB broth was supplemented with 10 mM MgCl2, indicating that expression of mgtA was repressed by high concentrations of Mg2+ (Fig. 2). Levels of β-galactosidase activity observed for cells producing either PhoQHis-T48S or PhoQHis-T48A were essentially similar to those obtained for cells producing wild-type PhoQHis, whether or not LB broth was supplemented with MgCl2 (Fig. 2). In contrast, mutations T48V and T48I in the PhoQHis protein increased the levels of β-galactosidase activity by 5-fold in the absence of MgCl2 and by 14- to 23-fold in the presence of 10 mM MgCl2. Although the PhoQHis-T48I and PhoQHis-T48V mutants responded to high concentrations of external MgCl2, levels of β-galactosidase activity produced by these cells grown in the presence of 10 mM MgCl2 were higher than the level of β-galactosidase activity obtained with cells producing wild-type PhoQHis grown in the absence of MgCl2. Interestingly, cells that produced the PhoQHis-T48L protein showed low levels of β-galactosidase activity whether or not LB broth was supplemented with 10 mM MgCl2 (Fig. 2). Similar results were obtained by measuring the β-galactosidase activity of the mgrB::lacZ transcriptional fusion (18) (data not shown). These data indicated that mutations T48V, T48I, and T48L in the PhoQHis protein affect the PhoP/PhoQ signaling cascade. As observed previously for mutation T48I (24), mutation T48V confers to cells a PhoP constitutively active phenotype. In contrast, mutation T48L appeared to barely respond to changes in the concentration of external MgCl2, conferring to cells a PhoP constitutively inactive phenotype.
FIG. 2.
Regulation of the mgtA::lacZ transcriptional fusion by the PhoQ-T48X mutants. (A) E. coli MG1607 cells carrying the pPROQHis-T48X plasmids were grown in LB broth or LB broth supplemented with 10 mM MgCl2. β-Galactosidase activities are expressed in Miller milliunits. Data are the means of triplicate values with standard deviations. WT, wild type; Ctl., control. (B) Western blot analysis of the expression level of the various PhoQ-T48X mutants.
In vitro global activity of the PhoP/PhoQ-T48X systems.
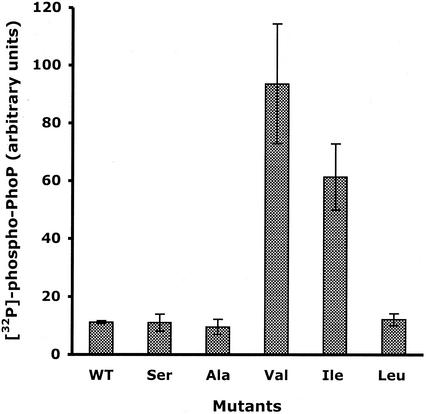
The level of phospho-PhoP inside the cell depends on both the autokinase and phosphatase activities of PhoQ. To assess in vitro the global activity of the PhoP/PhoQ-T48X systems, we reconstituted functional signaling pathways and measured the resulting net phosphorylation of PhoP. The various membranes containing the PhoQ-T48X proteins were incubated with a sixfold molar excess of PhoPHis in the presence of 5 mM MgCl2. Figure 3 shows the amounts of [32P]phospho-PhoPHis resulting from the catalytic activities of the various PhoQ-T48X proteins after a 20-min incubation period. For membranes containing the PhoQ-T48A, T48S, and T48L mutants, levels of radiolabeled PhoPHis were similar to those obtained with wild-type PhoQ (Fig. 3). Sixfold and ninefold increases in radiolabeled PhoPHis were observed with membranes containing the PhoQ-T48I and PhoQ-T48V mutants, respectively (Fig. 3). These data, consistent with our in vivo data shown in Fig. 2, confirmed that a Val or Ile residue at position 48 increases the net phosphorylation of PhoP. They also indicated that the Thr-to-Leu substitution did not affect the net phosphorylation of PhoP under these experimental conditions.
FIG. 3.
In vitro global activity of the PhoP/PhoQ-T48X regulatory systems. Membranes containing the PhoQ-T48X proteins were incubated at 22°C with a sixfold molar excess of PhoPHis in the presence of [γ-32P]ATP and 5 mM MgCl2. After 20 min, reactions (15 μl) were stopped by the addition of Laemmli SDS sample buffer. Aliquots were subjected to SDS-PAGE. The amount of 32P associated with the PhoPHis protein was quantitated with a PhosphorImager. Data are the means of triplicate values with standard deviations. WT, wild type.
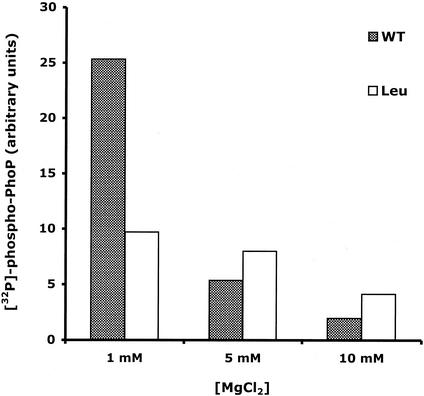
Influence of Mg2+ on the in vitro global activity of the PhoP/PhoQ-T48L system.
With the exception of the PhoQ-T48L protein, all the PhoQ-T48X sensors responded to changes in the concentrations of external MgCl2 (Fig. 2). To further investigate the apparent inability of this mutated protein to respond to MgCl2, we performed in vitro global assays at increasing concentrations of MgCl2. As shown in Fig. 4, increasing the concentration of MgCl2 from 1 to 10 mM decreased levels of radiolabeled PhoPHis by about 10-fold for wild-type PhoQ. In contrast, the PhoQ-T48L mutant only produced a twofold decrease in the level of radiolabeled PhoPHis (Fig. 4). These data indicated that the PhoQ-T48L sensor barely responds to the absence of Mg2+, suggesting that the Thr-to-Leu substitution locks the protein in a conformation that confers a PhoP constitutively inactive phenotype.
FIG. 4.
Influence of Mg2+ on the in vitro global activity of the PhoP/PhoQ or PhoP/PhoQ-T48L regulatory system. Membranes containing the PhoQ or PhoQ-T48L proteins were incubated at 22°C with a sixfold molar excess of PhoPHis in the presence of [γ-32P]ATP and increasing concentrations of MgCl2. After 20 min, reactions (15 μl) were stopped by the addition of Laemmli SDS sample buffer. Aliquots were subjected to SDS-PAGE. The amount of 32P associated with the PhoPHis protein was quantitated with a PhosphorImager. WT, wild type.
In vitro autokinase activity of the PhoQ-T48X sensors.
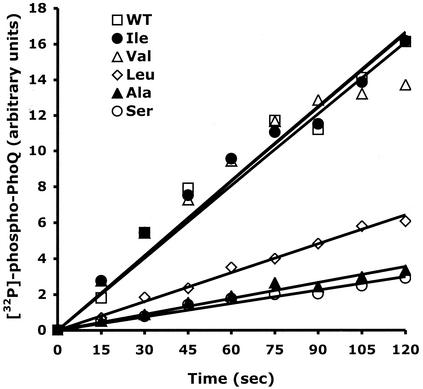
We wished to determine which of the individual PhoQ catalytic activities (autokinase and phosphatase) were affected by the mutations at position 48. We measured the initial rates of autophosphorylation of the various PhoQ-T48X sensors present in membranes. Using similar amounts of the various PhoQ-T48X proteins as judged by SDS-PAGE, autophosphorylation assays were performed as described in Materials and Methods. The PhoQ-T48I and PhoQ-T48V mutants were autophosphorylated at rates that are almost identical to that obtained for wild-type PhoQ (Fig. 5). In contrast, the Thr-to-Leu substitution (PhoQ-T48L) decreased the rate of autophosphorylation by about threefold (Fig. 5). Both the PhoQ-T48S and PhoQ-T48A mutants were autophosphorylated at rates that are fivefold slower than that obtained for the wild-type protein (Fig. 5). These data showed that the rates of PhoQ autophosphorylation vary significantly according to the nature of the substituting amino acid at position 48.
FIG. 5.
Initial rates of autophosphorylation of the PhoQ-T48X proteins. Membranes containing wild-type (WT) PhoQ (□), PhoQ-T48A (▴), PhoQ-T48S (○), PhoQ-T48V (▵), PhoQ-T48I (•), or PhoQ-T48L (◊) were incubated in the presence of [γ-32P]ATP and 0.1 mM mgCl2 for 2 min at 22°C. Reactions (15 μl) were stopped by the addition of Laemmli SDS sample buffer. Aliquots were subjected to SDS-PAGE. The amount of 32P associated with the PhoQ protein was quantitated with a PhosphorImager.
In vitro phosphatase activity of the PhoQ-T48X sensors.
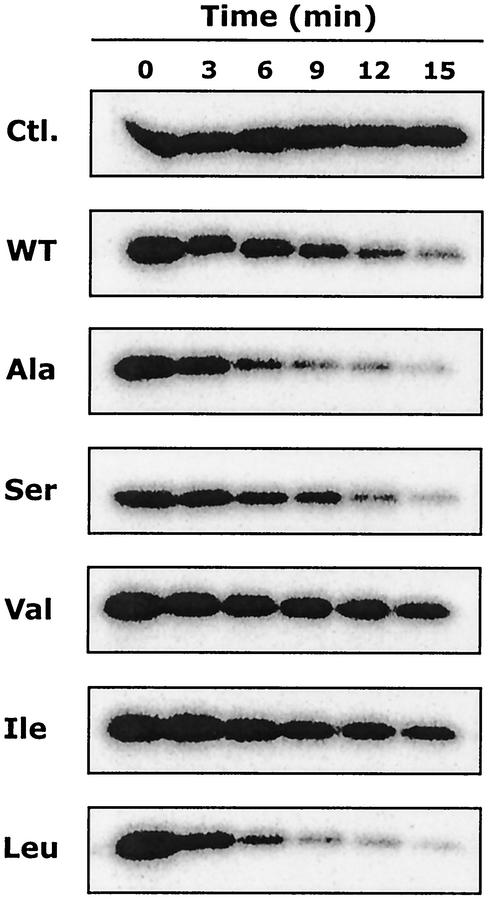
Substitutions at position 48 might affect not only PhoQ autokinase activity but also its phosphatase activity. Therefore, we compared the phosphatase activities of the various PhoQ-T48X sensors in vitro. Purified [32P]phospho-PhoPHis was incubated with membranes containing the various PhoQ-T48X proteins. Aliquots were removed at different time points, and the time course of the reaction was monitored as shown in Fig. 6. A control reaction in which [32P]phospho-PhoPHis was incubated with control membranes lacking PhoQ indicated that [32P]phospho-PhoPHis was stable during the reaction period. The phosphatase activities of PhoQ-T48I and PhoQ-T48V were decreased compared to that of wild-type PhoQ (Fig. 6). In contrast, the PhoQ-T48L protein and, to a lesser extent, the PhoQ-T48A protein showed increased phosphatase activities compared to wild-type PhoQ (Fig. 6). The phosphatase activity of PhoQ-T48S was essentially similar to that of wild-type PhoQ (Fig. 6). These data indicated that PhoQ phosphatase activity is also significantly affected by the various substitutions at position 48. Thus, we conclude that both the autokinase and phosphatase activities of PhoQ might be affected by the mutations at position 48.
FIG. 6.
Dephosphorylation of phospho-PhoPHis by the PhoQ-T48X proteins. [32P]phospho-PhoPHis was prepared and purified as described in Materials and Methods. Purified [32P]phospho-PhoPHis was incubated with either control membranes lacking PhoQ (Ctl.) or membranes containing PhoQ-T48X proteins. At indicated time points, aliquots (15 μl) were removed and reactions were stopped by the addition of Laemmli SDS sample buffer. Aliquots were subjected to SDS-PAGE. The amount of 32P associated with the PhoP protein was determined with a PhosphorImager. WT, wild type.
DISCUSSION
Previous studies have shown that a change of a Thr to Ile at position 48 of the PhoQ histidine kinase sensor (pho-24 allele of phoQ) results in a constitutively active PhoP/PhoQ regulatory system in S. enterica serovar Typhimurium (10, 14, 24). In our study, we examined the influence of various amino acid substitutions (Ala, Ser, Val, Ile, and Leu) at position 48 of PhoQ on signal transduction by PhoP/PhoQ. We identified three distinct classes of mutations, two of which altered expression of the mgtA::lacZ transcriptional fusion (Fig. 2).
The first class of mutations is represented by the mutations T48A and T48S. These mutations did not significantly alter the Mg2+-regulated expression of the mgtA::lacZ transcriptional fusion (Fig. 2). In vitro reconstitution of the PhoP/PhoQ-T48X systems showed that these mutations did not have an effect on the net autophosphorylation of PhoP (Fig. 3). However, analysis of the individual PhoQ catalytic activities showed that both mutations affected autokinase activity and, to a lesser extent, phosphatase activity (Fig. 5 and 6). Therefore, we conclude that in spite of differences in the individual activities, the balance between autokinase and phosphatase activity remained essentially unchanged for PhoQ-T48A and PhoQ-T48S. Interestingly, the side chains of Ala and Ser are smaller than that of the native Thr. Accordingly, we propose that small side chains or loss of the hydroxyl group at position 48 does not cause major structural alterations leading to important changes in the global activity.
The second class of mutations is represented by the mutations T48V and T48I (pho-24 allele of phoQ). Both mutations increased expression of the mgtA::lacZ transcriptional fusion and conferred a PhoP constitutively active phenotype (Fig. 2) (24). Both mutated proteins responded to increasing concentrations of Mg2+, albeit to a lesser extent than wild-type PhoQ (Fig. 2) (10). The increased net phosphorylation of PhoP observed for mutant T48V was essentially similar to that observed previously for the mutation T48I (Fig. 3) (14). By analyzing in vitro the individual autokinase and phosphatase activities, we found that PhoQ-T48V and PhoQ-T48I have decreased phosphatase activity whereas the rates of autophosphorylation were identical to that of wild-type PhoQ (Fig. 5 and 6). Thus, the PhoP constitutively active phenotype observed for the PhoP/PhoQ-T48V and PhoP/PhoQ-T48I systems is solely due to decreased phosphatase activity of PhoQ. For these mutated PhoQ sensors, the balance between autokinase and phosphatase activity shifted toward a kinase-dominant state. It is noteworthy that both Val and Ile are hydrophobic β-branched residues, whereas the native Thr is a polar β-branched residue. Importantly, only hydrophobic residues that were β branched but not γ branched conferred a PhoP constitutively active phenotype. These observations suggest that the hydrophobicity as well as the shape of the residue at position 48 might be critical for PhoQ transmembrane signaling.
The third class of mutations is represented by the mutation T48L, which confers a PhoP constitutively inactive phenotype. For this mutation, expression of the mgtA::lacZ transcriptional fusion was repressed irrespective of the presence or absence of Mg2+ (Fig. 2). When compared to wild-type PhoQ, limiting concentrations of Mg2+ had minimal effect on the net phosphorylation of PhoP measured in vitro (Fig. 4). We found that this phenotype resulted from both a decreased autokinase activity and an increased phosphatase activity (Fig. 5 and 6). Thus, the PhoQ-T48L sensor is in a phosphatase-dominant state irrespective of the presence or absence of Mg2+. Leu is a hydrophobic residue that is isomeric to Ile but is γ branched. Thus, the longer side chain length of the Leu residue at position 48 might provide a steric hindrance that is responsible for the PhoP constitutively inactive phenotype observed for the PhoP/PhoQ-T48L system. This substantiates our hypothesis that both the shape and the hydrophobicity of residue 48 are critical for PhoQ transmembrane signaling.
The residue at position 48 of PhoQ might influence signal transduction by directly affecting Mg2+ binding or by interfering with the Mg2+-induced conformational signal that is transmitted through the membrane bilayer. The direct involvement of residue 48 in Mg2+ recognition is most unlikely, because the response to increasing concentrations of external Mg2+ was essentially similar for four of the five mutated PhoQ proteins analyzed (Fig. 2). It appears more likely that residue 48 is an important structural determinant for transmitting the Mg2+-induced transmembrane signal. Our data support our hypothesis that the side chain of residue 48 is involved in the conformational changes responsible for the switch between the kinase-dominant state and the phosphatase-dominant state.
Residue 48 of PhoQ is located at the N terminus of the periplasmic domain in close proximity to the cytoplasmic membrane (Fig. 1A). In closely related PhoQ homologues, like the PhoQ proteins of other enterobacteria, the Thr residue at position 48 is strictly conserved (Fig. 1B). In the Pseudomonas aeruginosa and Xylella fastidiosa PhoQ homologues, the residue 48 consists of an Ile and a Leu, respectively (Fig. 1B). Our results show that these mutations affect signaling of the Salmonella PhoQ protein. It is possible that in the three-dimensional structure of these proteins, compensating mutations counterbalance the presence of an Ile or a Leu at position 48. Alternatively, it is possible that the nature of the residue at position 48 modulates the level of expression of PhoP-regulated genes. Note that the environmental signal sensed by some of these PhoQ homologues may be different than Mg2+ (12). In other histidine kinase sensors, the corresponding region has been shown to be important for either transmembrane signaling or ligand recognition. The highly conserved I box that includes residues 41 to 53 of various EnvZ homologues has been shown to be involved in the signal transduction mechanism (30). The P box of the NarQ and NarX sensors that corresponds to a stretch of 17 conserved amino acids has been shown to be required for nitrate sensing and/or transmembrane signaling (4, 5, 31). Although the corresponding region in PhoQ homologues is not as highly conserved as the I box or the P box (Fig. 1B), the location of position 48 implies that this region is important for transmembrane signaling.
Taken together, our data indicate that substitutions at position 48 of PhoQ affect the balance between autokinase and phosphatase activities. Our findings define the structural requirement at position 48 of PhoQ and suggest that this residue is critical for the transmission of the ligand-induced conformational signal through the membrane bilayer. Future studies will determine whether the conformational changes that involve residue 48 affect the dimeric interface of the protein or the structure of isolated subunits.
ADDENDUM
After this paper was submitted, Regelmann et al. (A. G. Regelmann, J. A. Lesley, C. Mott, L. Stokes, and C. D. Waldburger, J. Bacteriol. 184:5468-5478, 2002) reported a similar study of the E. coli PhoQ sensor kinase. Although the PhoQ proteins of S. enterica serovar Typhimurium and E. coli are 86% identical and exhibit a Thr at position 48, the PhoQ-T48I mutants display opposite phenotypes.
Acknowledgments
We thank Ryutaro Utsumi for providing strain MG1607. We thank J. A. Kashul for editorial support.
This work was supported by grant MT-15551 from the Canadian Institutes for Health Research and by a fellowship to H.L.M. from Fonds de la Recherche en Santé du Québec (FRSQ). S.S. was the recipient of an F. C. Harrison Fellowship from the Department of Microbiology and Immunology, McGill University.
REFERENCES
- 1.Alpuche-Aranda, C. M., E. L. Racoosin, J. A. Swanson, and S. I. Miller. 1994. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp. Med. 179:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behlau, I., and S. I. Miller. 1993. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J. Bacteriol. 175:4475-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castelli, M. E., E. García Véscovi, and F. C. Soncini. 2000. The phosphatase activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J. Biol. Chem. 275:22948-22954. [DOI] [PubMed] [Google Scholar]
- 4.Cavicchioli, R., R. C. Chiang, L. V. Kalman, and R. P. Gunsalus. 1996. Role of the periplasmic domain of the Escherichia coli NarX sensor-transmitter protein in nitrate-dependent signal transduction and gene regulation. Mol. Microbiol. 21:901-911. [DOI] [PubMed] [Google Scholar]
- 5.Chiang, R. C., R. Cavicchioli, and R. P. Gunsalus. 1997. “Locked-on” and “locked-off” signal transduction mutations in the periplasmic domain of the Escherichia coli NarQ and NarX sensors affect nitrate- and nitrite-dependent regulation by NarL and NarP. Mol. Microbiol. 24:1049-1060. [DOI] [PubMed] [Google Scholar]
- 6.Deng, W. P., and J. A. Nickoloff. 1992. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal. Biochem. 200:81-88. [DOI] [PubMed] [Google Scholar]
- 7.Dutta, R., L. Qin, and M. Inouye. 1999. Histidine kinases: diversity of domain organization. Mol. Microbiol. 34:633-640. [DOI] [PubMed] [Google Scholar]
- 8.Ernst, R. K., T. Guina, and S. I. Miller. 2001. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 3:1327-1334. [DOI] [PubMed] [Google Scholar]
- 9.Fields, P. I., E. A. Groisman, and F. Heffron. 1989. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 243:1059-1062. [DOI] [PubMed] [Google Scholar]
- 10.García Véscovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 11.Groisman, E. A. 1998. The ins and outs of virulence gene expression: Mg2+ as a regulatory signal. BioEssays 20:96-101. [DOI] [PubMed] [Google Scholar]
- 12.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groisman, E. A., E. Chiao, C. J. Lipps, and F. Heffron. 1989. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. USA 86:7077-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunn, J. S., E. L. Hohmann, and S. I. Miller. 1996. Transcriptional regulation of Salmonella virulence: a PhoQ periplasmic domain mutation results in increased net phosphotransfer to PhoP. J. Bacteriol. 178:6369-6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 16.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins, S., J. P. Kraehenbuhl, F. Schodel, A. Potts, D. Peterson, P. de Grandi, and D. Nardelli-Haefliger. 1995. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 63:3279-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato, A., H. Tanabe, and R. Utsumi. 1999. Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J. Bacteriol. 181:5516-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kier, L. D., R. M. Weppelman, and B. N. Ames. 1979. Regulation of nonspecific acid phosphatase in Salmonella: phoN and phoP genes. J. Bacteriol. 138:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Moual, H., T. Quang, and D. E. Koshland, Jr. 1998. Conformational changes in the cytoplasmic domain of the Escherichia coli aspartate receptor upon adaptive methylation. Biochemistry 37:14852-14859. [DOI] [PubMed] [Google Scholar]
- 21.Lesley, J. A., and C. D. Waldburger. 2001. Comparison of the Pseudomonas aeruginosa and Escherichia coli PhoQ sensor domains. Evidence for distinct mechanisms of signal detection. J. Biol. Chem. 276:30827-30833. [DOI] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, S. I., and J. J. Mekalanos. 1990. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 172:2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, S. I., W. S. Pulkkinen, M. E. Selsted, and J. J. Mekalanos. 1990. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect. Immun. 58:3706-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montagne, M., A. Martel, and H. Le Moual. 2001. Characterization of the catalytic activities of the PhoQ histidine protein kinase of Salmonella enterica serovar Typhimurium. J. Bacteriol. 183:1787-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pegues, D. A., M. J. Hantman, I. Behlau, and S. I. Miller. 1995. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol. Microbiol. 17:169-181. [DOI] [PubMed] [Google Scholar]
- 28.Soncini, F. C., E. García Véscovi, F. Solomon, and E. A. Groisman. 1996. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J. Bacteriol. 178:5092-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Velkinburgh, J. C., and J. S. Gunn. 1999. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect. Immun. 67:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waukau, J., and S. Forst. 1999. Identification of a conserved N-terminal sequence involved in transmembrane signal transduction in EnvZ. J. Bacteriol. 181:5534-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams, S. B., and V. Stewart. 1997. Discrimination between structurally related ligands nitrate and nitrite controls autokinase activity of the NarX transmembrane signal transducer of Escherichia coli K-12. Mol. Microbiol. 26:911-925. [DOI] [PubMed] [Google Scholar]