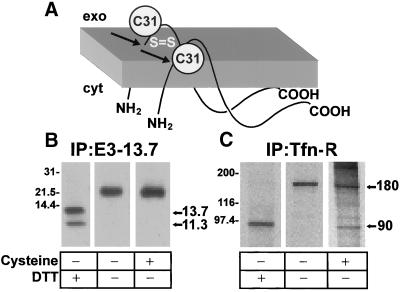
Figure 2.
The majority of E3-13.7 is protected from cell surface reduction at steady state. (A) Cartoon showing the membrane topology of E3-13.7 proteins (Hoffman et al., 1992a; Krajcsi et al., 1992). There are two distinct molecular mass species, a full-length 13.7-kDa protein and an 11.3-kDa protein cleaved cotranslationally at Ala-23 (arrows). Both species form disulfide-linked dimers at cysteine residue 31 (C31). (B and C) Infected cells were metabolically labeled for 1 h followed by a 3-h chase in nonradioactive medium to allow labeled proteins to achieve steady-state distribution. Some cells were incubated with a solution containing cysteine to reduce surface proteins with external disulfide bonds. Cell lysates were immunoprecipitated for E3-13.7 proteins (B) or the transferrin receptor (C). Immunoprecipitates were resolved by SDS-PAGE under reducing (+DTT) or nonreducing (−DTT) conditions. Sizes of reduced and nonreduced protein species are indicated to the right of each panel. E3-13.7 dimers have approximate molecular weights of 21,000–23,400 (Hoffman et al., 1992a). Additional molecular weight standards: phosphorylase B, 97,400; carbonic anhydrase, 31,000.