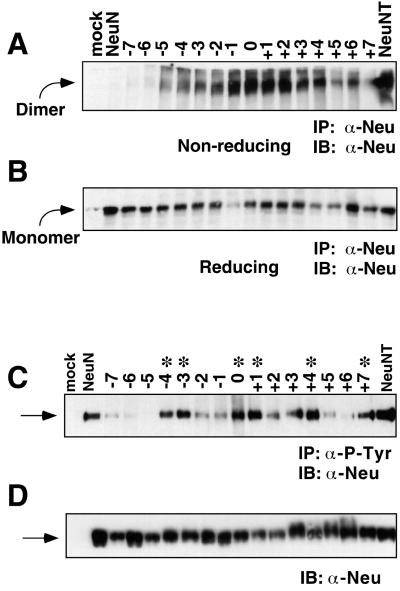
Figure 3.
(A) Dimerization of Neu TM-shift mutants assayed by SDS-PAGE electrophoresis under nonreducing conditions, or reducing conditions (B). Receptors were immunoprecipitated with mAB 7.16.4, run on a 4–12% gradient gel, transferred to nitrocellulose, immunoblotted with C-18 Neu antisera and visualized by ECL. With the exception of the −7 shift, −6 shift, and +7 shift mutants, all other mutants exhibit a significant percentage of the receptor present as a dimer. Note that in the immunoblot shown, recovery of the −1 sample was anomalously low for unknown reasons. This was not observed for the −1 sample in other repeats of this experiment. (C) Tyrosine phosphorylation of Neu TM-shift mutants. RIPA lysates immunoprecipitated with 4G10 anti-phosphotyrosine mAb were electrophoresed on a 4–12% gradient SDS-PAGE gel, transferred to nitrocellulose, immunoblotted for Neu by using C-18 Neu antisera, and detected by ECL. ∗, mutants that exhibit an increase in P-tyr incorporation, which coincides with transformation. (D) Lysates from C were immunoblotted with C-18 Neu antisera to demonstrate approximately equivalent expression of Neu in all samples. Arrows in C and D indicate monomeric Neu receptors.