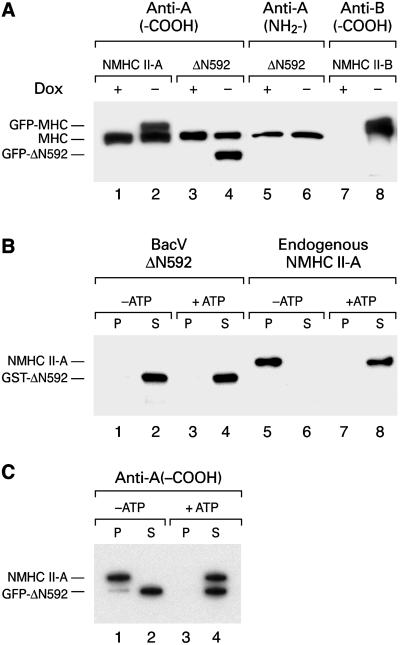
Figure 2.
Immunoblot analysis and actin-binding studies of NMHC-II and ΔN592. (A) Inducible expression of GFP-NMHC II-A, II-B, and GFP-ΔN592 in HeLa Tet-Off cells. The stable cell lines cultured with (+) or without (−) doxycycline (Dox) for 3 d were subjected to immunoblot analysis by using antibodies raised against amino acids at the carboxy terminus (anti-A, -COOH, lanes 1–4) or at the amino terminus (anti-A, NH2-, lanes 5 and 6) of the myosin II-A heavy chain as well as antibodies raised against the carboxy terminus (anti-B, -COOH, lanes 7 and 8) of the myosin II-B heavy chain. The HeLa Tet-Off cell line from Clontech does not contain endogenous NMHC II-B (lane 7), but introduction of a plasmid encoding NMHC-B into these cells by stable transfection resulted in NMHC II-B expression in the absence (lane 8), but not in the presence of Dox (lane 7). (B) Immunoblot demonstrating that ΔN592 does not bind to actin. Purified baculovirus-expressed ΔN592 does not cosediment with actin in the presence or in the absence of ATP (lanes 1 and 3). In contrast, endogenous NMHC II-A binds to actin in the absence of ATP (lane 5), but not in the presence of ATP (lane 8). P, pellet; S, supernatant. (C) GFP-ΔN592 does not dimerize with endogenous NMHC II-A. Cell extracts from HeLa cells expressing GFP-ΔN592 and endogenous NMHC II-A were incubated with F-actin in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of Mg2+-ATP, followed by sedimentation resulting in pellet (p) and supernatant (s) fractions. GFP-ΔN592 does not bind to actin in the absence or presence of ATP (lanes 2 and 4). Endogenous NMHC II-A bound to actin in the absence of ATP (lane 1). Note that only a very small amount of GFP-ΔN592 cosedimented with endogenous NMHC II-A (lane 1). This was most likely due to minor trapping of ΔN592 in the actin pellet.