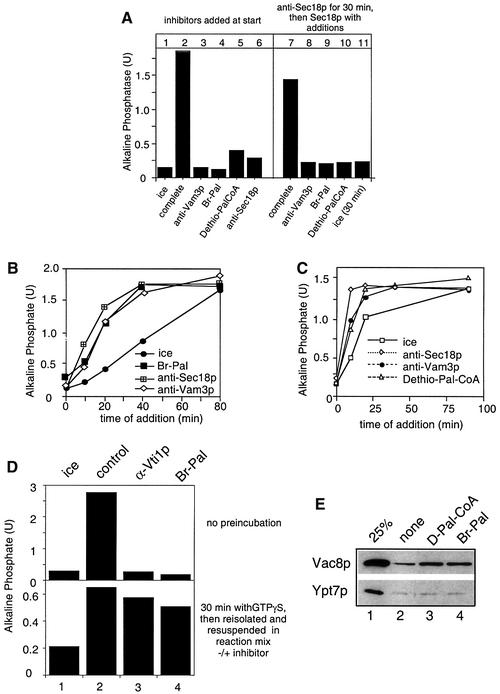
Fig. 4. Palmitoylation inhibitors block vacuole docking. (A) Requirement for palmitoylation cannot be fulfilled before Sec18p action. Vacuoles were incubated for 30 min at 26°C in the presence of ATP, cytosol and CoA and in the absence (lanes 1–6) or presence (lanes 7–11) of antibodies to Sec18p. The indicated inhibitors were added to the reactions shown in lanes 1–6 before the start. After 30 min, reactions 7–11 were placed on ice, diluted with 300 µl of PS buffer, centrifuged (8000 g, 4 min, 4°C) and resuspended in 30 µl of reaction buffer containing ATP, cytosol, CoA and 100 ng of Sec18p. Br-Pal (200 µM), dethio-Pal-CoA (30 µM) or antibodies to Vam3p were added where indicated. Incubations were for 90 min at 26°C. Then fusion was assayed. (B) Time-of-addition experiment with Br-Pal. A 30× scale fusion reaction was started in the presence of ATP, CoA (10 µM) and cytosol at 26°C. Aliquots (30 µl) were removed at the indicated time, antibodies (200 ng/µl) to Sec18p, Vam3p or Br-Pal (200 µM) were added and samples were incubated further at 26°C for a total of 90 min before being assayed for fusion activity. Ice = samples were placed on ice at the indicated times. (C) As for (B) except that dethio-Pal-CoA (30 µM) was added instead of Br-Pal. (D) The fusion step is insensitive to inhibitors of palmitoylation. Upper panel: fusion reactions (30 µl) containing ATP, cytosol and inhibitors [antibodies to Vti1p (200 ng/µl) or Br-Pal (200 µM) as indicated] were incubated for 90 min at 26°C or on ice. Lower panel: fusion reactions (30 µl) containing ATP, cytosol and 2 mM Mg-GTPγS were incubated at 26°C for 30 min. Samples were then diluted with 150 µl of PS buffer containing 150 mM KCl, and vacuoles were reisolated (3 min, 8000 g, 4°C) and resuspended in 30 µl of reaction buffer containing ATP, cytosol and the inhibitors as indicated. Fusion was determined after an additional 60 min incubation at 26°C or on ice. (E) A block in palmitoylation causes release of Vac8p from the vacuole. BJ3505 vacuoles (15 µg) were incubated in a 75 µl reaction for 30 min at 26°C in the presence of ATP, and Br-Pal (200 µM) or dethio Pal-CoA (30 µM) where indicated. Vacuoles were then reisolated (10 min, 8000 g, 4°C) and proteins in the reaction supernatant were precipitated by 13% trichloroacetic acid (v/v). Proteins were then analyzed by SDS–PAGE and immunoblotting with antibodies to Vac8p and Ypt7p. Bands were quantified by laser densitometry. In lanes 2–4, release to the supernatant was 4.3, 12 and 11% for Vac8p, and 2.6, 3.1 and 2.7% for Ypt7p, respectively.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.