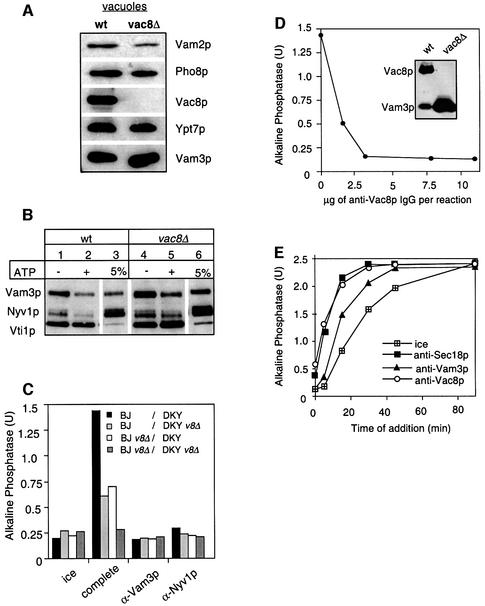
Fig. 7. Vac8p is essential for fusion. (A) Protein composition of vacuoles from wild-type or vac8Δ mutants. Vacuoles (10 µg) from DKY6281 and the corresponding deletion mutant were solubilized in SDS sample buffer, boiled for 4 min at 95°C and analyzed by SDS–PAGE and western blotting. The immunoblot was decorated with antibodies to Vam3p, Vac8p, Pho8p, Vam2p and Ypt7p. (B) Deletion of Vac8p does not influence the integrity of the SNARE complex. Vacuoles from BJ3505 or the corresponding strain lacking Vac8p were purified, incubated for 10 min in the presence or absence of ATP and processed for co-immunoprecipitation with anti-Vti1p antibodies as described in Figure 6A. (C) Fusion of vac8Δ vacuoles. Vacuoles from the tester strains and the respective vac8Δ strains were incubated for 90 min at 26°C in the presence of cytosol, Sec18p (20 ng), CoA (10 µM) and ATP in the combinations shown. Inhibitors such as IgGs to Vam3p or Nyv1p (both at 200 ng/µl) were added where indicated. After the incubation, fusion was assayed as described. v8Δ stands for vac8Δ. (D) Sensitivity of vacuole fusion to antibodies against Vac8p. Fusion reactions (30 µl) containing vacuoles from both tester strains, ATP and cytosol were incubated for 90 min at 26°C. Increasing amounts of purified IgGs against full-length Vac8p (see Materials and methods) were added where indicated. After 90 min, fusion was measured. The inset shows an immunoblot of vacuoles (wt = 5 µg, vac8Δ = 50 µg) decorated with Vac8p (new) and Vam3p antibodies. (E) Vac8p antibodies inhibit at priming. Reactions were incubated and aliquots removed as described in Figure 4B, except that IgGs to Sec18, Vam3p and Vac8p (200 ng/µl) were added as inhibitors.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.