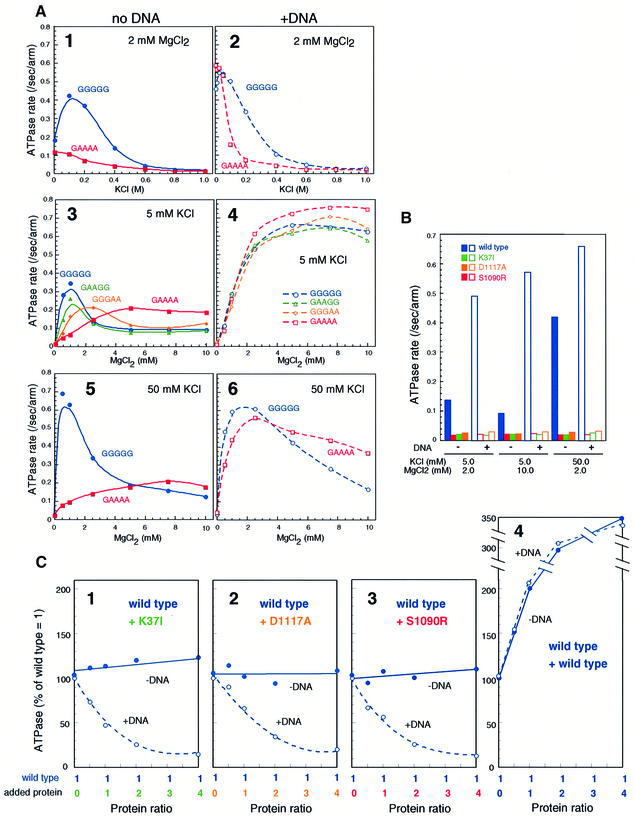
Fig. 5. Two distinct modes of ATPase activation in the absence or presence of DNA. (A) KCl titration at 2 mM MgCl2 (panels 1 and 2), MgCl2 titration at 5 mM KCl (panels 3 and 4), and MgCl2 titration at 50 mM KCl (panels 5 and 6) in the absence (panels 1, 3 and 5) or presence (panels 2, 4 and 6) of φX174 virion DNA (31.2 μM nucleotides). A fixed protein concentration of 300 nM arms (equivalent to 150 nM dimers in the case of wild-type BsSMC) was used for all the proteins. The rate of ATP hydrolysis is expressed as the number of ATP molecules hydrolyzed per second per arm. (B) ATPase-defective mutants. The ATPase rate of four different proteins (wild type, K37I, D1117A and S1090R; 300 nM arms for each) was determined under different KCl/MgCl2 concentrations in the absence (solid bars) or presence (open bars) of φX174 virion DNA (31.2 μM nucleotides). (C) Mixing ATPase assay. A fixed concentration of wild type (300 nM arms) was mixed with increasing concentrations (0–1200 nM arms) of K37I (panel 1), D1117A (panel 2), S1090R (panel 3) or wild type (panel 4). The ATPase activity of the mixtures was determined in the absence (filled circles) or presence (open circles) of φX174 virion DNA (31.2 μM nucleotides). The concentrations of KCl and MgCl2 were 50 and 2 mM, respectively. The activity is shown by percentage of ‘wild-type ratio = 1 (300 nM arms)’. At the highest concentration of wild-type protein [panel 4; total protein ratio = 5 (1500 nM arms)], the rate of ATP hydrolysis is saturated and is no longer proportional to the input amount of protein.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.