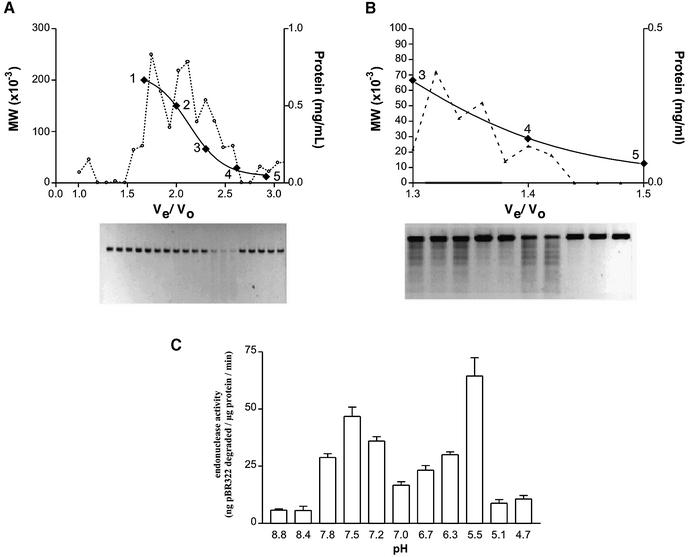
Figure 1.
Size exclusion chromatography and endonuclease activity of salt-extracted mitochondrial inner membrane proteins. Separation of proteins extracted from the inner mitochondrial membrane was achieved by means of size exclusion chromatography. The elution of known standards was used to identify the approximate size of the proteins in the active fractions: 1, β-amylase (200 kDa); 2, alcohol dehydrogenase (150 kDa); 3, bovine serum albumin (66 kDa); 4, carbonic anyhdrase (29 kDa); 5, cytochrome c (12.4 kDa). The relative activity of each fraction was determined by the loss of 20 ng of linearized pBR322 incubated in 20 µl of buffer containing proteins extracted from the inner mitochondrial membrane at a concentration of 0.02 mg/ml. (A) Sephacryl HR-200 separation; proteins were incubated with DNA for 1 h at 37°C. (B) Sephacryl HR-100 separation; proteins and DNA were incubated for 10 min at 37°C. (C) pH dependence of endonuclease activity.