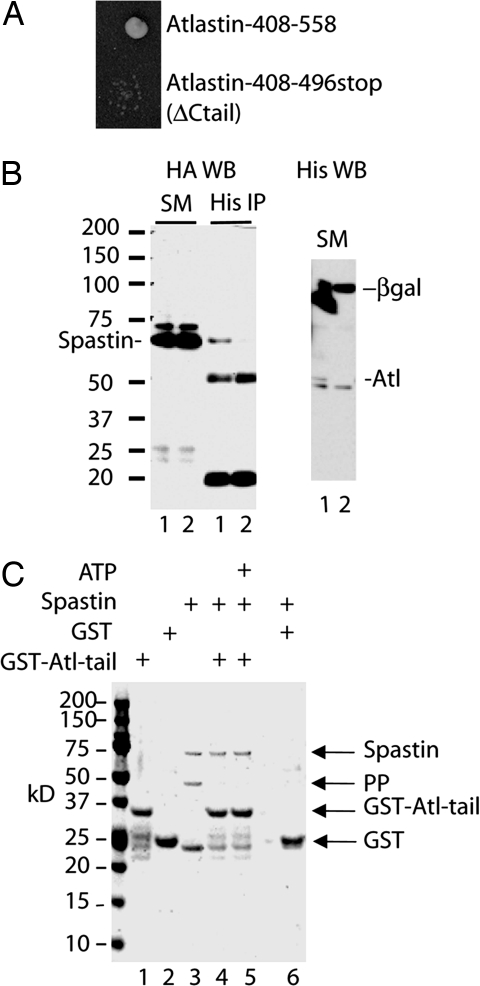
Fig. 1.
Spastin–atlastin interaction. (A) A yeast two-hybrid screen identified atlastin as a spastin interactor. Full-length human spastin was used as a bait and interacted with a clone encoding amino acids 408–558 of human atlastin-1. This clone and a deletion mutant lacking the C-tail of atlastin (ΔCtail) were used to retransform yeast in the two-hybrid assay. The original clone, but not the ΔCtail clone, interacted with spastin in this assay. (B) Coimmunoprecipitation of spastin and atlastin. HeLa cells were transiently transfected for 24 h with 2HA-spastin and either atlastin-His-6x (lysate 1) or His-6x-tagged β-gal (lysate 2) as a negative control. Cell lysates were subjected to immunoprecipitation with a His-6x antibody followed by anti-HA (Left) or anti-His-6x (Right) Western blotting. Both lysates expressed equivalent amounts of HA-spastin (SM) and spastin coimmunoprecipitated with atlastin but not β-gal. The 50- and 20-kDa bands are Ig heavy and light chains. (C) Coomassie blue-stained gel showing that spastin binds to the atlastin C-tail. The GST was cleaved from GST-spastin with precision protease (PP), and the crude reaction mixture (lane 3) containing 10 μg of spastin was applied to glutathione-Sepharose beads containing 10 μg of GST-atlastin C-tail or GST alone (lanes 4–6) in a total volume of 200 μl. Lanes 1 and 2 show samples of the beads containing only GST-atlastin C-tail or GST. All of the added spastin but none of the cleaved GST or PP bound to the atlastin C-tail, and ATP was not required for binding (compare lanes 4 and 5). In contrast to GST-spastin, the GST-atlastin C-tail protein does not contain a PP cleavage site.