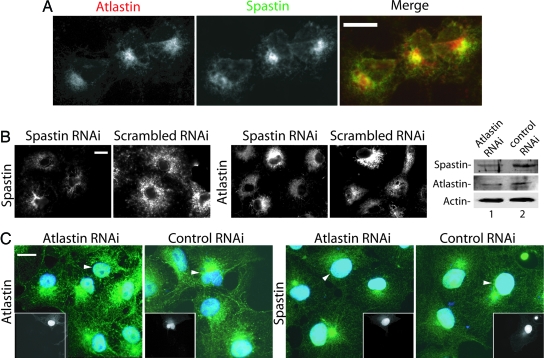
Fig. 4.
Colocalization of endogenous spastin and atlastin. (A) Cos cells were stained with antibodies to atlastin and spastin. Signals were detected by using a Cy-5-conjugated or Alexa Fluor 488 secondary antibody. Spastin signal was detected by using Alexa Fluor 488. Note the partial colocalization of the two proteins. Atlastin staining intensity was variable, and the spastin signal is higher in cells expressing more atlastin. (B) Spastin RNAi does not appreciably alter atlastin staining. Spastin expression (Left) was partially suppressed by transfection of a spastin-specific siRNA but not a scrambled siRNA. Transfection with a rhodamine-labeled siRNA showed that >90% of the cells are transfected (data not shown). Duplicate coverslips prepared from the same transfections were stained for endogenous atlastin (Right), which appeared unaltered. (C) Effects of atlastin RNAi on spastin. Cells were fixed 48 h after transfection, and duplicate coverslips were stained for either spastin or atlastin. In this experiment, background staining of nuclei was sometimes observed. Transfected cells, identified by GFP expression (Insets), are indicated by arrowheads. Nuclei were stained with DAPI (blue). Compared with nontransfected cells, atlastin staining is partially suppressed in atlastin RNAi but not in control RNAi cells. Compared with control transfected cells, a smaller percentage of cells subjected to atlastin RNAi showed the perinuclear concentration of spastin (29% vs. 45%, n = 135 cells from each transfection). Quantitative Western blot analysis of equal numbers of transfected cells obtained by flow cytometry demonstrates that atlastin suppression was 60% and that spastin levels were also reduced to a similar degree. Signals were normalized to actin intensity. (Scale bars, 15 μm.)